Abstract
The RNA-binding protein LIN28B regulates developmental timing and determines stem cell identity by suppressing the let-7 family of microRNAs. Postembryonic reactivation of LIN28B impairs cell commitment to differentiation, prompting their transformation. In this study, we assessed the extent to which ectopic lin28b expression modulates the physiological behavior of neural crest cells (NCC) and governs their transformation in the trunk region of developing embryos. We provide evidence that the overexpression of lin28b inhibits sympathoadrenal cell differentiation and accelerates NCC migration in two vertebrate models, Xenopus leavis and Danio rerio. Our results highlight the relevance of ITGA5 and ITGA6 in the LIN28B-dependent regulation of the invasive motility of tumor cells. The results also establish that LIN28B overexpression supports neuroblastoma onset and the metastatic potential of malignant cells through let-7a-dependent and let-7a-independent mechanisms.
Similar content being viewed by others
Introduction
Neural crest cells (NCC) undergo substantial modifications in their morphology and function during embryogenesis. Their highly migratory and invasive nature allows them to reach distant body districts, whereas their pluripotency assures the specification of several cell lineages, including peripheral neurons and the adrenal medulla [1, 2]. The initial migratory wave of NCC is activated by the epithelial-to-mesenchymal transition (EMT). The morphological changes triggered by EMT sustain cell migration from the neural tube, invasion of the surrounding tissues, and spatial progression toward the peripheral sites, where cells differentiate and unfold their intrinsic features [3]. Several NCC characteristics, such as motility, polarity, invasiveness, and plasticity, are largely shared by a number of highly metastatic tumors, including colon [4], breast [5], esophagus [6], and neuroblastoma [7] tumors. Neuroblastoma is an embryonal malignancy responsible for ~15% of cancer-related deaths during childhood [8]. It originates from the transformed sympathoadrenal cell precursors deriving from trunk NCC [9] and is characterized by vast clinical, genetic, and biological heterogeneity [10]. About half of patients with neuroblastoma are stratified as high-risk (HR), having an overall survival rate of <40% despite intensive multimodal therapy [11]. The main feature of HR patients with neuroblastoma is the prevalence of metastases at disease onset. The metastases to the liver, the bone marrow, the bone, or lymph nodes are found in ~50% of HR patients. Like other pediatric cancers, neuroblastoma shows a low frequency of somatic mutations although alterations in several genes, including LIN28B, have been associated with this malignancy [12].
The RNA-binding proteins LIN28A/LIN28B were initially identified as important regulators of developmental timing [13]. By inhibiting let-7 microRNA biogenesis and through direct binding of the target RNAs, LIN28 regulates numerous cellular activities that are essential for embryogenesis [14], but it shows protumorigenic features if maintained beyond the physiologically defined timeframe [15, 16]. In neuroblastoma, the protumorigenic function of LIN28B has been attributed to either gene amplification or overexpression [7]. However, the lack of experimental models, in which modulated levels of LIN28B can be studied during early developmental phases, limits the possibility for a comprehensive investigation of the mechanisms that sustain NCC transformation.
In this study, we assessed the role of the ectopically expressed zebrafish lin28b gene in regulating trunk NCC migration and differentiation toward the sympathoadrenal lineage. In two vertebrate models, zebrafish (Danio rerio) and Xenopus (Xenopus leavis), we examined how the overexpression of lin28b affected the migration of trunk NCC during early embryonic development. We then analyzed whether lin28b determined the differentiation of NCC toward noradrenergic lineage. In vivo, a stable overexpression of the human LIN28B gene driven by the dβh promoter was adopted to evaluate the probability of neuroblastoma onset. In the tumor cells, we focused on evaluating the effects of the prolonged overexpression of LIN28B on cell motility and dissemination in vitro and in the in vivo xenograft model. Finally, we established the relevance of integrin-dependent signaling in the regulation of neuroblastoma cell migration upon LIN28B overexpression.
Results
Lin28b overexpression impairs the differentiation of sympathoadrenal precursor cells
To estimate the effects of lin28b overexpression during embryonic development, we injected capped lin28b mRNA into 1–2-cell stage zebrafish embryos. We then assessed the ectopic expression of the corresponding transcript and protein at different developmental stages (Fig. S1A) compared with the control (ctrl) embryos, injected with either the mCherry or the GFP mRNAs (Fig. S1B). Wild-type uninjected embryos were used as a blank control for fluorescence screening, and as a reference sample for the evaluation of Lin28b protein levels in the immunoblot assay (Fig. S1B). Of note, the injected fish showed no overt macroscopic phenotypes or developmental failures (Fig. S1C). We then verified the functionality of Lin28b by observing a significant reduction of the let-7a expression in lin28b embryos (Fig. S1D). To test if Lin28b affected the development of sympathoadrenal neurons, we analyzed the expression of tyrosine hydroxylase (th) and dopamine β-hydroxylase (dβh) as hallmarks of NCC differentiation toward the sympathoadrenal lineage. The ectopic expression of lin28b at early stages of development led to a marked reduction of both dβh and th mRNAs in the superior cervical ganglia (SCG) as compared with GFP-injected controls (Fig. 1a). Moreover, TH protein levels also significantly decreased upon lin28b overexpression (Fig. 1b). In addition, lin28b-injected embryos expressed lower levels of the SCG marker zash1a (Fig. 1c), a transcription factor required for early sympathoadrenal cell specification [17]. These findings imply the involvement of lin28b overexpression in the loss of prodifferentiating signaling in sympathoadrenal cells already at early stages of embryonic development. Then, to verify the evolutionary conservation of Lin28b function in the tetrapods, we performed transient gain-of-function experiments on the Xenopus embryos. In this model, it is possible to specifically target the central nervous system and NCC without affecting the development of other tissues [18]. We therefore injected lin28b mRNA into one dorsal blastomere at the four-cell stage (Fig. 1d, left panel) along with GFP mRNA in order to select and further analyze only embryos overexpressing lin28b in the developing central nervous system. Comparable with the effects observed previously in the zebrafish, the injected lin28b mRNA caused a significant reduction of the sympathoadrenal marker reptin in the Xenopus embryos (Fig. 1d, right panel). To assure that the observed reduction of sympathoadrenal cells in lin28b embryos was not the result of damaged cell proliferation or induced apoptosis, we stained the SCG with either EdU or activated caspase-3/TUNEL, respectively. We found no significant differences in the number of proliferating TH+ cells in the SCG of injected embryos (Fig. 1e). Similarly, caspase-3 and TUNEL stainings showed no relevant activation of apoptosis in the SCG of lin28b larvae (Figs. 1f and S2). These results confirm that lin28b overexpression determines the failure of sympathoadrenal progenitor cell differentiation toward their functional counterparts without affecting their proliferation or viability.
Lin28b overexpression causes sympathoadrenal cell loss. a In situ hybridization for the progenitor markers dβh and th in the superior cervical ganglia (dashed squares) of the control (ctrl) and lin28b embryos at 80 hpf. Scale bar: 100 μm. The fraction of embryos displaying the corresponding phenotype is provided in each panel. b The western blot analysis of the indicated proteins in the control (ctrl) and lin28b embryos at 72 hpf. The molecular weights are indicated in kilodaltons (KD). Protein quantification is shown in the lower panel. The error bars represent SEM. **p < 0.01. c Labeling of zash1a mRNA in 72 hpf control (ctrl) and lin28b zebrafish embryos. Dashed circles highlight the developing superior cervical ganglia. The fraction of embryos displaying the corresponding phenotype is provided in each panel. d The schematic experimental overview is presented on the left side. On the right side, a representative example of a Xenopus embryo (out of 76 embryos analyzed) at stage 35 is shown, indicating the expression of the sympathoadrenal marker reptin in the developing adrenal medulla (dashed squares). The lateral view of the lin28b injected side (right image) and the uninjected side (ctrl side, left image) of the same embryo is presented. e Proliferation of sympathoadrenal progenitors identified by tyrosine hydroxylase (TH) immunolabeling (red) coupled with EdU incorporation (green) in the control (ctrl) and lin28b embryos at 80 hpf. The quantification is shown in the right panel (n = 30 embryos for each group). n.s.: p > 0.05. Scale bar: 100 μm. f The control (ctrl) and lin28b embryos were stained for activated caspase-3 (green) and TH (red) to detect apoptosis in the superior cervical ganglia at 80 hpf. Scale bar: 100 μm
Impaired cell differentiation promotes neuroblastomagenesis in LIN28B-overexpressing zebrafish
To assess whether the lin28b-dependent block of peripheral sympathetic neural cell differentiation has protumorigenic effects in fish, we generated the stable transgenic zebrafish line Tg(dβh: hLIN28B), referred to here as hLIN28B. For this purpose, human LIN28B was overexpressed in the peripheral sympathetic nervous system under the control of the dβh promoter. Tumor masses arose in the anterior abdomen of 6-month-old transgenic animals (Fig. 2a) with a 2.4% penetrance. The tumors were found in the interrenal gland, a common site of neuroblastoma onset [19], and consisted of small, undifferentiated, round tumor cells with distinct single nuclei (Fig. 2b). To molecularly characterize and assure the tumor type, we analyzed the expression of several neuroblastoma-specific markers. The tumor cells were strongly immunoreactive for TH (Fig. 2c), indicating their peripheral sympathetic neuronal origin [20]. They were also positive for the HuC/D and the Synaptophysin markers (Fig. 2c), demonstrating that they arose from sympathetic neuroblast precursors, like human neuroblastomas, and not from other NCC derivatives, such as chromaffin cells. Together, these findings evince that the overexpression of human LIN28B in the peripheral sympathetic neuronal system of transgenic fish promotes neuroblastomagenesis with a penetrance below 5%, approximately as described for human patients with neuroblastoma.
LIN28B-induced neuroblastoma in zebrafish. a Macroscopic views of the control (ctrl) and hLIN28B zebrafish at 6 months of age, showing a tumor mass found in the anterior abdomen (white dashed line). b Haematoxylin–Eosin (H&E) staining of the sagittal sections of the tumors in the interrenal gland (IRG) of 6-month-old fish. Scale bar: 100 μm. c Immunohistochemical analyses of the sagittal sections of the Tg(dβh:GFP) control (ctrl) animals and the hLIN28B transgenic fish using tyrosine hydroxylase (TH), HuC/D, and Synaptophysin antibodies. Nuclei were counterstained with hematoxylin (purple). Scale bar: 100 μm
Overexpressed lin28b increases the migratory capacity of trunk NCC
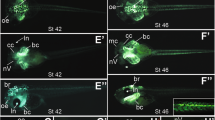
The different number of sympathoadrenal cells composing the SCG between the lin28b and control fish reflects the possible involvement of mechanisms regulating either NCC migration or their specification during the early phases of functional commitment. To assess this hypothesis, we used the Tg(sox10:GFP) zebrafish transgenic line [21]. In these fish, GFP expression is regulated by the NCC promoter sox10, allowing the real-time tracking of the NCC inside developing embryos. Transient overexpression of lin28b influenced the migration of trunk NCC, as confirmed by live cell imaging, without influencing the total number of GFP+ cells (Fig. S3A), their proliferation and viability (Fig. S3B, C). Interestingly, although NCC from both the control and lin28b embryos migrated ventrally along somites in narrow stripes as expected (Fig. 3a and Supplementary Video S1A), the NCC overexpressing lin28b were first to reach their destination toward the dorsal aorta (Fig. 3a and Supplementary Video S1B). To quantify this phenomenon, we measured the migration distances of NCC labeled with crestin riboprobe (Fig. 3b) [22]. The average NCC migration distance was significantly longer in lin28b embryos compared with the controls at both monitored time points (Fig. 3c). Importantly, the same behavior was also evident in the Xenopus embryos, where lin28b-overexpressing sox10+ NCC cells [23] reached longer distances in the lin28b versus the control side of the embryo within the same timeframe (Fig. 3d). Together, these data prove that Lin28b regulates the migration of trunk NCC in different vertebrate models without affecting their proliferation and survival rates. Following, to test how effective Lin28b was in determining the sympathoadrenal lineage specification, we assessed the capability of NCC to form cell lineages other than the sympathoadrenal lineage. First, we analyzed the development of melanocytes originating from trunk NCC [24]. The amount of melanocytes in the yolk sac and the yolk extension was unaffected upon lin28b overexpression (Fig. 3e). Moreover, a comparable number of sensory neurons composing the trunk NCC-derived dorsal root ganglia (DRG) was found in both control and lin28b larvae (Fig. 3f). Furthermore, the DRG developed in the middle of the single somite boundary, as expected [25]. We then looked at the enteric neurons deriving from vagal NCC [26]. Upon staining with the anti-HuC/D antibody, no significant differences in the number of enteric neurons were found between the control and lin28b larvae (Fig. 3g). The finding that lin28b embryos lacked trunk NCC-derived sympathoadrenal precursor cells but maintained vagal NCC-derived neurons indicates that Lin28b may play different roles in the designation of specific subpopulations of trunk NCC.
Lin28b increased the migration speed of trunk NCC. a Sequential images of a time-lapse movie in Tg(sox10:GFP) embryos showing the motility of NCC in lin28b embryos (white arrows) and controls (ctrl). The reported frames were recorded at 19 hpf (T0) after 109 min (T109) and 292 min (T292). Scale bar: 200 μm. b The representative scheme depicting the analysis of zebrafish trunk NCC migration. The migrated distance of NCC (purple) toward the dorsal aorta (dotted line) in the same region of the trunk (from somite 8 to somite 12) of embryos injected with the GFP mRNA (ctrl) or with lin28b mRNA (lin28b) has been analyzed. c A total of 50 injected embryos per group were stained with the crestin riboprobe at 19 hpf (upper panel) and 24 hpf (lower panel), displaying the migration of trunk NCC toward the ventral region of the notochord (dotted black lines). The quantification of the percentage of the migrated distance is shown in the graph. *p < 0.05. Scale bar: 100 μm. d The trunk sox10+ NCC of the uninjected (ctrl; left image) and lin28b injected (right image) sides of the Xenopus analyzed at stage 30 (out of the 89 embryos analyzed). A migrated distance of NCC (black square brackets) toward the dorsal aorta is depicted. e Imaging of the control (ctrl) and lin28b embryonic pigment patterns at 48 hpf showed melanocytes populating the yolk sac and the equal yolk extension (dashed squares) between the two groups (the average number of melanocytes in controls = 29 ± 4 and lin28b embryos = 28 ± 5, p-value = 0.31). f The HuC/D antibody staining (green signal) revealed the presence of segmentally arranged dorsal root ganglia in the trunk of both the controls (ctrl) and lin28b embryos at 4 dpf (the average number of HuC/D+ cells in controls = 20 ± 2 and lin28b embryos = 22 ± 3, p-value = 0.2). For individual cell counts composing the zebrafish dorsal root ganglia (DRG), the five most caudal DRG labeled with the HuC/D antibody were counted for each condition (n = 30 embryos per group). g The number of HuC/D-positive enteric neurons (green-positive nuclei) remained comparable between the lin28b embryos compared with the control (ctrl) siblings at 6 dpf (the average number of HuC/D+ cells in controls = 36 ± 4 and lin28b embryos = 33 ± 5, p-value = 0.4. n = 30 embryos per group). Scale bar: 100 μm
Overexpression of LIN28B increases the migratory capacity of SH-SY5Y cells
To functionally test the observed in vivo correlations between lin28b overexpression and the increased NCC migratory phenotype, we overexpressed LIN28B in the neuroblastoma cell line SH-SY5Y under a short-term (48 h) and a long-term (7 days and 14 days) period. LIN28 is known to be a master regulator of pluripotency in embryonic stem cells [23, 27]. Gene expression analysis indicated that 48 h of LIN28B overexpression significantly increased the levels of the stemness-associated genes Oct4, Sox2, and Nestin (Fig. 4a) while preserving colony formation and cell proliferation potentials upon the long-term expression of LIN28B in SH-SY5Y cells (SH-SY5YLIN28B) and the controls (SH-SY5YCTRL) (Fig. S4A, B). Importantly, while LIN28B and let-7a expression patterns showed reciprocal expressions after short-term LIN28B induction, the let-7a levels reverted after prolonged overexpression (Fig. 4b). These findings imply that intervals of LIN28B overexpression define molecular mechanisms that can be both let-7a-dependent and let-7a-independent. Long-term LIN28B overexpression provoked consistent changes in cell morphology (Figs. 4c and S4C), which was accompanied by a notable reorganization of cytoskeleton microfilaments (Figs. 4d and S4D). Indeed, SH-SY5YCTRL cells formed small and stellate-shaped cell clumps, whereas SH-SY5YLIN28B cells were bigger and cuboidal in shape. Given the role of cytoskeleton reorganization in cell motility [28] and the observed higher migratory rates of NCC upon lin28b overexpression in vivo, we analyzed the correlation between these phenomena in vitro. Ectopic LIN28B significantly increased the migratory properties of SH-SY5YLIN28B cells, as revealed by scratch and transwell assays (Fig. 4e, f). A detailed analysis of cell movement using time-lapse imaging showed a sharp increase in total migrated distances (Figs. 4g and S4E; Supplementary Movies S2A and S2B) and in the mean cell velocities of SH-SY5YLIN28B cells upon 7 days of LIN28B induction (Fig. 4h). Notably, motility continued to progress upon LIN28B induction for 14 days (Fig. S4F–H; Supplementary Movies S3A and S3B). These results provide further evidence that LIN28B overexpression is a determinant factor for defining the migration of neural crest-derived cells both in vitro and in vivo.
The Overexpression of LIN28B in SH-SY5Y cells potentiates their migratory phenotype. a The qPCR analysis showed a significantly increased expression of the stemness markers Nestin, Sox2, and Oct4 upon 7 days [d] of LIN28B overexpression. The results show the average of three biological replicate experiments. GAPDH was used as the internal control. The error bars represent SEM. **p < 0.01, *p < 0.05. b The let-7a level was evaluated at several time points upon LIN28B induction (48 hours [h], 7 and 14 d) through qPCR analyses. The results display the mean values of the relative expression ± error bars representing SEM. **p < 0.01; n.s.: p > 0.05. c The morphology of the SH-SY5YCTRL and SH-SY5YLIN28B cells treated with doxycycline for 7 d. Scale bar: 100 μm. d Phalloidin (F-Actin) staining (green) of the SH-SY5YCTRL and SH-SY5YLIN28B cells upon 0 h and 7 d of doxycycline treatment. Scale bar: 50 μm. e The SH-SY5YCTRL and SH-SY5YLIN28B cells were examined for their migratory capacity in a wound healing assay upon 7 d of doxycycline administration. Representative photographs are reported in the left panel. The percentage of wound closure at every time point was calculated relative to the wound width at 0 h (right graph bars). n.s.: p > 0.05; **p < 0.01. Scale bar: 100 μm. f The transwell migration assay on SH-SY5YCTRL and SH-SY5YLIN28B cells upon 7 d of doxycycline administration. Migrated cells were stained with DAPI (blue) and cristal violet (insets) and counted in following for ten separated fields. **p < 0.01. Scale bar: 100 μm. g The cell motility tracks of SH-SY5YCTRL and SH-SY5YLIN28B cells (n = 15 cells tracked) treated with doxycycline for 7 d. The red dots indicate the final cell positions on their path. The distances are presented in μm. h The dot plot reports the average cell speed (μm/min) of the SH-SY5YCTRL and SH-SY5YLIN28B cells upon 7 d of doxycycline administration. p = 1.4 × 10−3
LIN28B promotes the invasive motility of neuroblastoma cells
The core feature of highly aggressive tumors is their metastatic potential determined by enhanced cell migration and invasion abilities. Both features are also mandatory for the proper function of NCC during embryogenesis. To investigate whether LIN28B may sustain cell invasiveness, we analyzed the capacity of SH-SY5YLIN28B cells to infiltrate the matrigel-coated Boyden chambers (Fig. 5a, upper panel). This assay confirmed an increased capacity of SH-SY5YLIN28B cells to invade the basement membrane following a chemo-attractant gradient when compared with the control SH-SY5YCTRL counterpart (Fig. 5a, graph bars). To confirm these findings in vivo, we injected either SH-SY5YLIN28B or SH-SY5YCTRL cells into the duct of Cuvier of Tg(fli1:GFP) zebrafish embryos with blood vessels marked in green. While SH-SY5YLIN28B cells showed a rapid spread inside the vessels and clear distribution throughout the trunk of the embryos 1-day post injection, SH-SY5YCTRL cells did not disseminate in the same way (Fig. 5b). This proinvasive phenotype was sustained by EMT induction, as SH-SY5YLIN28B cells expressed significantly higher levels of the mesenchymal markers N-cadherin, Snail, Twist1, and Slug compared with the control cells upon long-term LIN28B induction (Figs. 5c and S5A). Consistently, the levels of N-cadherin, Snail, Twist1, and Slug genes were also significantly upregulated after LIN28B ectopic expression (Fig. 5d). These findings explained the previously observed changes in SH-SY5YLIN28B cell morphology (Fig. 4d) that aligned well with the mesenchymal-like phenotype. In line with these outcomes, the overexpression of lin28b in the zebrafish embryos led to a global activation of EMT (Fig. 5e–g) as well. Taken together, the results imply that LIN28B is sufficient to trigger EMT and to increase the invasive capacities of neural crest-derived neuroblastoma cells.
LIN28B activates EMT in neuroblastoma cells and increases their invasive potential in vivo. a The assessment of transwell migration was determined 24 h after seeding the SH-SY5YCTRL and SH-SY5YLIN28B cells pretreated with doxycycline for 7 days. The quantification of migration was determined by measuring the mean fluorescence intensity, and the results are presented on the histogram. **p < 0.01. b Fluorescent microscopy images of the trunk region of the Tg(fli1:GFP) zebrafish embryos with marked vessels (green) injected at 48 hpf with SH-SY5YCTRL and SH-SY5YLIN28B cells (red signal) 7 days upon treatment with doxycycline. Visualizations of cells at 4 h post implantation (hpi) and at 1 day post implantation (dpi) are depicted. White arrowheads indicate cells disseminated throughout the trunk vasculature. n = number of animals analyzed. Scale bar: 100 μm. c The western blot analysis for the indicated EMT markers on SH-SY5YCTRL and SH-SY5YLIN28B upon 7 days of doxycycline. β-Tubulin was used to show equal protein loading. The molecular weights are indicated in Kilodaltons (KD). d The overexpression of LIN28B for 7 days increased the expression levels of the mesenchymal markers Twist1, Snail, N-cadherin, and Slug. GAPDH was used as an internal control. **p < 0.01. e qPCR analysis for the indicated EMT markers in the ctrl and lin28b-overexpressing zebrafish embryos at 48 hpf. The results are normalized to the gapdh internal control. The error bars represent SEM. **p < 0.01. The western blot analysis of 72 hpf (f) and 96 hpf (g) zebrafish embryos extracts for the indicated EMT markers and Lin28b protein. β-Actin was used as a loading control. The molecular weights are indicated in Kilodaltons (KD). The quantification of the same is shown in the right graph bars. *p < 0.05; **p < 0.01
LIN28B activates integrin-signaling pathways
The migration of trunk NCC during body segmentation and somitogenesis is mediated by their interaction with the extracellular matrix (ECM) components. Integrins are a family of transmembrane receptors required for cell adhesion and their migration [29,30,31]. To investigate whether the acquired migratory and invasive potentials of LIN28B-overexpressing neuroblastoma cells could be attributed to integrin signaling, we evaluated the gene expression patterns of a number of integrins. Out of the nine transcripts analyzed, the most apparent upregulation was found for two integrins, α5-integrin (ITGA5) and α6-integrin (ITGA6). Both of these showed a substantial induction upon prolonged LIN28B overexpression (Fig. 6a). Accordingly, the expression of the corresponding proteins, ITGA5 (CD49e) and ITGA6 (CD49f), was triggered in SH-SY5YLIN28B cells (Figs. 6b and S5B–D) and confirmed by flow cytometry analyses (Fig. 6c, d). Then, to assess the importance of the two transmembrane proteins in the regulation of SH-SY5YLIN28B cell motility, we examined their migration upon the addition of antagonizing primary antibodies. Pretreatment with either the blocking antibodies anti-ITGA5 (ITGA5ab) or anti-ITGA6 (ITGA6ab) successfully abolished the migration of LIN28B-overexpressing cells (Fig. 6e, f and Supplementary Videos S5–S8). Correspondingly, cell speed was dramatically reduced only in ITGA5ab- and ITGA6ab-treated SH-SY5YLIN28B cells, suggesting that the previously described promigratory phenotypes were largely hooked up by the two cell-surface integrins in LIN28B-overexpressing cells. Because the integrin clustering is essential for the early phases of cell adhesion to the substrate, we next assessed if the observed phenotype was associated with altered focal adhesions’ (FAs) formation. While the SH-SY5YCTRL cells exhibited a diffuse cytosolic distribution of FA adapter proteins Paxillin, focal adhesion kinase (FAK), and Vinculin [32], the SH-SY5YLIN28B cells possessed these proteins organized on the plasma membrane as punctate, peripheral adhesions (Figs. 7a and S6A). At day 7, these modifications were sustained mainly by the activation of two integrin/FAK-related signaling routes, including PI3K p85/AKTSer473 and Src pathways (Figs. 7b and S6B). Both signaling cascades are important players in regulating the integrin-based cell motility [33, 34]. However, at day 14 only ERK activation was detected (Fig. S6C), implying for a dynamic temporal regulatory switch between multiple signaling proteins in LIN28B-overexpressing cells. Taken together, our observations attribute to LIN28B a novel role in orchestrating the integrin-mediated cell spreading and migration.
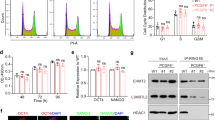
Ectopic expression of LIN28B triggers integrin expression and increases cell motility potential. a qPCR analysis for ITGAs (ITGA1-6, 11) and ITGBs (ITGB1, 4) upon 7 days of LIN28B overexpression. GAPDH was used as an internal control gene. n.s.: *p < 0.05; **p < 0.01. b Immunofluorescence on SH-SY5YCTRL and SH-SY5YLIN28B cells upon 7 days of doxycycline administration for ITGA5 (green, upper panel) and ITGA6 (lower panel) protein deposition (white arrows). The nuclei were counterstained with DAPI (blue). Scale bar: 50 μm. Insets correspond to higher magnification of the ITGA5/6-positive cells. The flow cytometry profile of SH-SY5YCTRL and SH-SY5YLIN28B cells over 7 days of doxycycline administration, showing c ITGA5-positive (CD49e, p = 0.05, left panel) and d ITGA6-positive (CD49f, right panel, p = 0.04) cells. e The cell motility tracks of SH-SY5YCTRL and SH-SY5YLIN28B cells treated with doxycycline for 7 days and incubated with a non-neutralizing IgG control antibody or with the blocking antibody (ab) against ITGA5 (ITGA5ab; 12 cells tracked) prior to the time-lapse imaging. The red dots indicate the final position of the cell migration paths. The corresponding average cell speeds of SH-SY5YCTRL and SH-SY5YLIN28B are depicted in the box plots. IgG plot: p = 3.1 × 10−6; ITGA5 plot: p = 1 × 10−1. f The cell motility tracks of SH-SY5YCTRL and SH-SY5YLIN28B cells treated with doxycycline for 7 days and incubated with a nonneutralizing IgG control antibody or the blocking antibody (ab) against ITGA6 (ITGA6ab; 12 cells tracked) prior to the time-lapse imaging. The red dots indicate the final position of the cell migration paths. The corresponding average cell speeds of SH-SY5YCTRL and SH-SY5YLIN28B are depicted as box plots. IgG plot: p = 6 × 10−4; ITGA6 plot: p = 8.9 × 10−1
Ectopic expression of LIN28B triggers focal adhesion reorganization and activation of integrin/FAK-related signaling pathway. (a) The immunostaining of the focal adhesion (FA) associated adapter proteins Paxillin, FAK, and Vinculin (green) in combination with Phalloidin (F-Actin, red) is presented. Local redistribution of the proteins in the FAs is indicated by white arrowheads and evidenced as insets. The nuclei were counterstained with DAPI (blue). FAs of 5 cells within 3 random fields were counted, and data were presented as mean ± SEM from 3 independent experiments. **p < 0.01. (b) The Western blot analysis for the indicated markers on SH-SY5YCTRL and SH-SY5YLIN28B upon 7 days of doxycycline administration. An antibody against β-Tubulin was used as a loading control. The molecular weights are indicated in Kilodaltons (KD)
Discussion
The highly conserved RNA-binding protein LIN28B is expressed in developing tissues and is required for proper embryogenesis [13]. One of the most recognized roles of LIN28B involves the inhibition of the tumor suppressing let-7 family of microRNAs [14], contributing to the maintenance of cell pluripotency. The altered expression of LIN28B has been reported in various types of human cancer [4, 35, 36] while its role as an oncogene has been confirmed in different mouse models [7, 37]. In neuroblastoma, genomic amplification or overexpression of LIN28B are associated with the HR group of patients for whom the widespread metastatic disease is regularly present [7]. Nevertheless, LIN28B-driven mechanisms of tumorigenesis are still poorly characterized for neuroblastoma, especially because the onset of this tumor occurs during the early embryonic developmental stages.
In this study, we assessed the in vivo effects of fish lin28b overexpression on the NCC compartment. We relied on the zebrafish and Xenopus vertebrate models to demonstrate that the ectopic expression of lin28b led to a block in the differentiation of NCC-derived sympathetic progenitor cells without affecting their proliferation or vitality. We showed that Lin28b is required for the commitment of trunk NCC toward the sympathoadrenal cell lineage but is dispensable for the delineation of other NCC derivatives, such as pigmented cells and enteric neurons. This raises the possibility that Lin28b-derived signaling may act in specific NCC subpopulations in which the overexpression of lin28b beyond its physiological timeframe is sufficient for the malignant transformation of trunk sympathoadrenal cell precursors and for neuroblastoma’s onset. In fact, the transgenic zebrafish model with dβh-driven overexpression of human LIN28B caused tumors in six-month-old fish. These tumors showed high immunohistochemical similarities with human neuroblastomas and with previously reported neuroblastomas developed in zebrafish [38]. More importantly, the penetrance of LIN28B-positive tumors in fish aligned to the one previously described for human neuroblastomas. We then studied the molecular consequences of enforced short-term and long-term overexpression of the LIN28B gene in vitro in NCC-derived neuroblastoma cells. We could distinguish the short-term and long-term effects of LIN28B overexpression as respectively let-7a-dependent and let-7a-independent, sustaining previous findings [39]. Moreover, the LIN28B-driven acquisition of migratory and invasive properties was accompanied by substantial morphological changes and the increase of several mesenchymal markers, sustaining LIN28B as a promoter of EMT. During normal embryonic development, NCC undergo EMT and the subsequent migration is largely dependent on the cell-ECM crosstalk. Integrins are composed of diverse heterodimeric transmembrane receptors transducing the signals bidirectionally and regulating cell adhesion, migration, and invasion [40]. In our study, we identified a significant increase of two integrins, ITGA5 and ITGA6, upon long-term LIN28B overexpression. The importance of ITGA6 in inducing metastasis has been reported in several cancer types diagnosed in adults [41, 42], whereas in neuroblastoma, its involvement in cell dissemination was not previously described. Conversely, ITGA5 induction has been recently associated with a metastatic neuroblastoma phenotype [43]. Consistent with previous findings, our results highlight the importance of integrin-mediated prometastatic behavior in LIN28B-overexpressing neuroblastoma cells. They also sustain the role of LIN28B in facilitating a dynamic remodeling of cell adhesion proteins [44]. The recruitment of FA clusters on the plasma membrane of LIN28B-overexpressing cells implies their value for sustaining the migratory properties of tumor cells. In this scenario, the activation of downstream pathways at first involved the PI3K/AKT and Src kinases, followed by a delayed ERK engagement. All of them were described along with a shift toward more motile phenotypes and a favored FA formation [45]. These results imply that LIN28B overexpression promotes mesenchymal-like phenotypes whose motility is allowed by the Integrin/FAK axis and dynamic regulation of several intracellular pathways. Moreover, the striking expression of ITGA5/ITGA6 on the SH-SY5YLIN28B cell membrane may be critical for shaping the interaction between the ECM and tumor cells and, hence, for their motility and invasion capacities. Both characteristics are required for tumor cells’ dissemination and metastasis formation. Our findings specify additional roles of LIN28B in the regulation of the interaction between neuroblastoma cells and their surrounding niche. Previous reports affirm the abundance of fibronectin and laminin, high-affinity binding targets of ITGA5 and ITGA6, within the trunk region of the zebrafish embryos [46, 47]. Their neutralization provokes the inhibition of NCC migration, pointing out the relevance of both ECM molecules for the proper function of NCC [47]. Hence, hLIN28B-overexpressing zebrafish could allow not only novel understandings of the cell-ECM interactions but may also serve as an in vivo model for evaluating the effects of selective integrin inhibitors in the treatment of metastatic neuroblastoma.
In conclusion, we confirmed the oncogenic potential of LIN28B and its association with neuroblastoma’s onset in the zebrafish model. We showed a positive correlation between LIN28B overexpression and the induction of EMT, a process that led to the cell morphology transformation and increment of invasive traits of tumor cells. We additionally highlighted the complexity of the LIN28B-dependent pathways not necessarily involving let-7a miRNA. Further, we described the association between LIN28B overexpression and the prometastatic phenotype of neuroblastoma cells. This feature was triggered by activating ITGA5 and ITGA6 expression and by the accumulation of corresponding proteins on the cell membrane. Together, our results suggest that LIN28B sustains integrin-mediated cell spreading and migration by facilitating the formation of focal contacts. The two integrins, ITGA5 and ITGA6, could therefore be new molecular markers in prometastatic neuroblastoma. Further investigation on whether they might be potential targets in a group of HR patients remains to be performed.
Materials and methods
Animals
Wild-type (AB/TU) zebrafish [48] and the transgenic line Tg(sox10:GFP) [21] were staged and maintained as described previously [49]. All experiments using animal models were approved by the local Ethical Committees and by the Italian Ministry of Health (zebrafish authorization 86/2016-PR; Xenopus authorization 99/2012-A). Xenopus embryos were obtained and staged as described previously [17].
Cell lines
SH-SY5Y cells were purchased from the DSMZ (Germany) and grown in RPMI-1640 (Sigma-Aldrich) with the addition of 2 mM penicillin/streptomycin (Gibco), 2 mM l-glutamine (Gibco), and 10% fetal bovine serum (FBS; Gibco). Cell line genotyping was conducted prior to the analyses.
SH-SY5YCTRL and doxycycline-inducible SH-SY5YLIN28B cell lines were obtained by a lentiviral infection; pLenti CMV CTRL Blast plasmid (Addgene #17492) and modified pLenti CMV/TO GFP Puro plasmid (GFP was replaced by LIN28B ORF, Addgene, #17481) were used. Lentiviral particles were produced by co-transfecting either the CTRL or LIN28B plasmids with the packaging vector psPAX2 (Addgene #12260) and the envelope plasmid pMD2.G (Addgene #12259) into HEK-293T cells (ICLC) in the Opti-MEM culture medium (Gibco) with 1 mg/ml polyethylenimine (Sigma-Aldrich). The cells stably expressing the TetR control gene (SH-SY5YCTRL) were grown in 7 μg/ml of Blasticidin-S HCl (Gibco) enriched selecting medium. A population of SH-SY5YLIN28B cells was generated upon growth in 2 μg/ml of puromycin (Gibco) enriched selective medium.
Capped mRNA overexpression
The SP6 Message machine kit (Ambion) was used to transcribe synthetic capped RNA. Zebrafish embryos were injected with 50–100 pg of lin28b or GFP mRNA at the 1–2-cell stage. Injected embryos were raised from 16 h post fertilization (hpf) to 6 days post fertilization (dpf) and fixed with 4% paraformaldehyde for further analysis. Xenopus embryos were injected with lin28b or GFP mRNA in one blastomere at the four-cell stage, and then fixed with 4% paraformaldehyde for further analysis.
Constructs and probes
The pCS2+-mCherry construct was a kind gift of Dr Enrico Moro (University of Padua). The pCS2+-GFP was generated from the pME-GFP construct from the Multisite Gateway-based construction kit (Tol2Kit) [50]. Zebrafish lin28b cDNAs were obtained by PCR and cloned into the pCS2+ vector. The pcDNA3-FLAG-LIN28B construct used for the doxycycline-inducible SH-SY5Y cell line was a kind gift from Narry Kim (Addgene plasmid #51373). The following antisense RNA probes were also generous gifts: dbh, th [51], and zash1a [52]. The crestin probe was generated by amplifying the zebrafish ORF from the wild-type whole embryo cDNA, and the PCR product was cloned into the pCR-II-TOPO vector (Invitrogen). Following sequence verification, antisense riboprobes were generated by in vitro transcription with a DIG RNA labeling kit Sp6/T7 (Roche). Antisense riboprobes were also generated following sequence verification through in vitro transcription with a DIG RNA labeling kit Sp6/T7 (Roche). The dre-let-7a probe was purchased from EXIQON. The Xenopus pCS2+-lin28b plasmid was generated by RT-PCR and fully sequenced. The plasmids used for the preparation of reptin and sox10 antisense RNA probes are described elsewhere [53, 54].
Transgenesis
The 5.2-kb promoter region of the dβh gene was PCR amplified from the zebrafish BAC clone CH211-270H11 using the primers dβh-fw (#599): GCG TAC TCC CCC TTT TTA GG and dβh-rev (#600): TGT TGC TTT GTC GTC TTT TGA. The PCR product was cloned into the gateway p5-MCS vector (a kind gift of the Chien lab) using the KpnI/XhoI restriction sites. LIN28B was isolated from pcDNA3-Flag-LIN28B (Addgene #51373) by BamHI/NotI digestion and cloned into the gateway middle entry vector pENTR1A using the same restriction enzymes. The full transgenesis construct was gateway assembled [50] to generate the pDEST5.2dβh-LIN28B-CG2. The embryos were injected with this DNA construct at the one-cell stage and grown to adulthood. Fin clips from the offspring were used for the genotyping of stable integration and the germline transmission of the transgene. The Tg(dβh: hLIN28B) was designated as the “hLIN28B” transgenic line in this article.
In situ hybridization
Whole-mount RNA in situ hybridization of zebrafish and Xenopus embryos was performed, as previously described [17, 55]. Briefly, fixed embryos were permeabilized with 10 μg/ml of Proteinase K and, after 5 h of preincubation with Hybridization Mix (50% Formamide, 5X SSC, 0.01% Tween-20, 50 μg/ml of heparin, 500 μg/ml of t-RNA), they were incubated with 100 ng/μl of the antisense probe at 65 °C. The second day, embryos were extensively washed and incubated in a Blocking Solution (2% sheep serum, 2 mg/ml of BSA in PBS/0.1% Tween-20) for 2 h, followed by the addition of the Anti-Digoxigenin antibody (Anti-Digoxigenin-AP Fab fragment, Roche), diluted 1:3000. The embryos were washed several times in PBTw, followed by the incubation with the Staining Solution containing NBT/BCIP (Roche). Stained embryos were sectioned and mounted in 80% glycerol, and images were acquired with a Carl Zeiss Axio microscope.
Immunofluorescence
Immunofluorescence of whole zebrafish embryos was performed essentially as described [56]. Fixed embryos were permeabilized in cold acetone for 7 min at −20 °C, followed by three washes in PBTx (1% Triton X-100 in 1× PBS) for 5 min at RT in agitation. The embryos were blocked in 3% goat serum diluted in PBTx for 1 h in agitation, and primary antibodies were then added overnight at 4 °C. The embryos were washed three times in PBTx for 10 min and incubated with secondary antibodies in 5% goat serum/PBTx in the dark for 3 h. Lastly, the embryos were washed two times in PBTx for 10 min and transferred in low melting 1.5% agarose (Sigma-Aldrich) and mounted in slides, and images were acquired with a Carl Zeiss Axio microscope and a Leica TCS-SP5-II confocal microscope. Primary and secondary antibodies are listed in Supplementary Table S1.
SH-SY5Y cells were fixed using 4% PFA in PBS for 15 min before permeabilization with 0.1% Triton X-100 diluted in PBS. To reduce the background fluorescence, the coverslips were blocked in 3% BSA prepared in PBS for 1 h at room temperature. Cells were stained with phalloidin-TRITC, ITGA5, and ITGA6 antibodies. The cells in Fig. S5C were incubated with 3% BSA without cell permeabilization. The proteins were visualized with a Zeiss LSM 800 confocal microscope. For Paxillin, FAK, and Vinculin staining, the coverslips were fixed and permeabilized as previously described. The coverslips were subsequently treated with primary antibodies for 1 h and after PBS washing with a secondary antibody in a dark humid chamber for 45 min. The coverslips were subsequently mounted on glass slides and observed by confocal microscopy (Zeiss LSM 800 confocal microscope). The number of FAs per cell was assessed using ImageJ software.
Western blot
Zebrafish-derived proteins were extracted from pools of 30 embryos through mechanical desegregation in Lysis Buffer (Tissue Extraction Reagent I, Life Technologies) supplemented with 1% Protease/Phosphatase Inhibitor Cocktail (Sigma-Aldrich). The proteins from neuroblastoma cells were extracted with commercially available lysis buffer (Biosource International) from the samples pre-treated with doxycycline, as described previously [57].
Samples were centrifuged at 14,000 × g for 5 min at 4 °C, and the protein concentration was determined using the bicinchoninic assay (BCA) method (Pierce BCA Protein Assay Kit, Thermo Scientific). A total amount of 50 µg (zebrafish) and 20 µg (cell lines) of proteins were loaded in the CriterionTM TGX Stain-FreeTM Precast Gels 4–20% (Bio-Rad), and then transferred on the nitrocellulose membrane (Midi-size LF PVDF Membrane, TransBlot®TurboTM, Bio-Rad, activated in 100% Methanol) through a semidry transfer with the Trans-Blot® TurboTM Transfer System (Bio-Rad). Membranes were blocked with Tropix® I-BLOCK (Thermo Fisher Scientific) for 2 h, followed by incubation with primary antibodies at 4 °C. The membranes were washed and incubated with secondary antibodies. Uvitec Cambridge was used for chemiluminescence detection, covering the membrane with ECL (ECL SelectTM Western Blotting Detection Reagent, GE Healthcare). The primary and secondary antibodies are listed in Supplementary Table S1.
EdU proliferation and TUNEL cell death assays
A proliferation assay was performed using a commercial kit, EdU-Click 488, as recommended by the manufacturer (Baseclick). Briefly, three independent pools of 15 living embryos were collected in microcentrifuge tubes, and 1 mM EdU was added, followed by incubation on ice for a variable time depending on their developmental stage. The embryos were washed with cold E3 medium on ice and then incubated for 30 min at 28.5 °C. After that, embryos were fixed with 4% paraformaldehyde for 2 h and permeabilized in 10 μg/ml Proteinase K. To detect proliferating cells, embryos were incubated in the Reaction Cocktail containing 10 mM of Dye Azide for 3 h. In following, the embryos were used for the coupled immunostainings to detect the SCG or the NCC. Cell death assay was performed using a commercial kit, Click-iT Plus TUNEL Assay, following manufacturer instruction (Life Technologies). Fixed embryos were permeabilized with 10 μg/ml Proteinase K and subsequently incubated with an Equilibration Buffer (TdT reaction buffer) for 1 h. Next, the embryos were incubated with the TdT enzyme for 90 min at 37 °C and then with the Reaction Cocktail for 30 min at 37 °C. The embryos were fixed again in 4% paraformaldehyde for 30 min, and the immunofluorescence with the anti-GFP antibody was then performed (see Supplementary Table S1). The embryos were then mounted in 3% methylcellulose, and images were acquired through a Zeiss LSM 800 confocal microscope.
Fluorescent cell labeling, embryo preparation, and tumor cell implantation
After 7 days of doxycyclin administration, SH-SY5YCTRL and SH-SY5YLIN28B cells were labeled with the Vybrant® DiI Cell-Labeling Solution (Invitrogen) according to the manufacturer’s instructions. The dechorionized (2 dpf) zebrafish embryos were anaesthetized with 0.003% tricaine (Sigma-Aldrich) and positioned on a 10 cm Petri dish coated with 3% agarose. Approximately 200 cells were injected within the duct of Cuvier of the anesthetized Tg(fli1:GFP) embryos using borosilicate glass capillary needles (OD/ID: 1.0/0.75 mm, WPI), a Pneumatic Picopump, and a micromanipulator (WPI). After implantation, zebrafish embryos were maintained at 33 °C. Embryos showing less than 30–40 cells after 4 h post injection were discarded from the analysis. At least 30 embryos per group were analyzed from three independent experiments. The embryos were live photographed using a Nikon C2 H600L confocal microscope (×20 water dipping objective).
Time-lapse
Tg(sox10:GFP) embryos were injected with LIN28B mRNA and allowed to develop for 19-20 hpf. Anesthetized embryos were embedded in 1.5% agar, mounted in a heated chamber, and imaged with a Zeiss Axio Observer microscope for live cell imaging for 8 h. Quantification of displacement was measured in a 40 µm Z-stack, tracked using Fiji software. The quantification of the percentage of the migrated distance relative to Fig. 3b was the measurement of the straight-line distance from the first position (neural tube) to the last position labeled with crestin riboprobe, with a higher number indicative of more distance between the first and last point. Statistical analysis was conducted in Excel followed by a two-tailed Student’s t-test. Neuroblastoma cells were analyzed in a 37 °C heated chamber with 5% CO2. Cell motility was captured over a 12-h period at 10-min intervals, and tracks were recorded using the Fiji “manual tracking” plug-in. For functional integrin blocking, the attached cells were preincubated for 30 min before migration analysis either with 10 µg/ml of mouse anti-human-ITGA6 or mouse anti-human-ITGA5 antibodies in 24-well plates (see Supplementary Table S1). The appropriate IgG antibodies (Mouse IgG2b, Millipore; Mouse IgG1, Santa Cruz Biotechnology) were used as negative controls. Cell motility was captured over a 12-h period at 10-min intervals.
The Wilcoxon test was used to assess the p-values quantifying the significance of the differences. All statistical analyses were performed using the R statistical software “stats” package.
Flow cytometric analyses
Zebrafish single-NCC were obtained from the pool of 40 embryos. Tg(sox10:GFP) and wild-type zebrafish embryos were dissected to remove the head and washed, and the tails were mechanically desegregated by constant pipetting in Hanks’ buffer (EMD Millipore Corporation). Single-NCC were obtained using Trypsin-EDTA (EMD Millipore Corporation) and Collagenase/Dispase (Sigma-Aldrich). After resuspending the NCC in Hanks’ buffer, analyses were performed through Cytomics FC 500 (Beckman Coulter). Wild-type uninjected embryos were used as blanks.
SH-SY5YCTRL and SH-SY5YLIN28B cells (0.3–0.5 × 106 cells) were incubated with PeCy5 anti-human CD49f (ITGA6, Becton Dickinson) and analyzed using a CytoFLEX Flow cytometer (Beckman Coulter). The percentage of CD49e (ITGA5) positive cells was validated upon coupled primary (1 µg) and secondary antibody labeling (see Supplementary Table S1). The percentage of CD49e- and CD49f-positive cells in both SH-SY5YCTRL and SH-SY5YLIN28B cells were considered by setting an appropriate gate on the living cell population (Propidium iodide negative, 10 mg/ml, Roche). Unlabeled cells for each line were first acquired to ensure labeling specificity.
Colony formation assay
Two thousand cells were seeded in a 24-well plate using a MethoCult semisolid medium (Stemcell Technologies) and were grown for 2 weeks. Colonies (foci) were visualized after incubation with MTT. The total colony number was counted under a light microscope in ten random fields and determined by Fiji software.
Transwell migration assay
SH-SY5YCTRL and SH-SY5YLIN28B cells were seeded onto inserts without Matrigel (BD Biosciences) in a serum-free medium. Inserts were placed into wells containing a medium supplemented with 10% FBS. After 24 h, the cells on the upper surface of the filters were removed, and the inserts were stained with Hoechst (Sigma-Aldrich) and cristal violet. The numbers of migrated cells were counted under a light and a fluorescent microscope in ten random fields. Using Fiji software, the number of migrated cells per image was determined.
Matrigel invasion assay
SH-SY5YCTRL and SH-SY5YLIN28B cells (5 × 104) were seeded onto matrigel-coated transwell chambers (BD Biosciences) with an 8-µm pore size filter using an FBS gradient (0–10%) from the top to the bottom well. After 24 h of incubation, the filter was removed and washed, and the cells that invaded the lower side of the filter were stained with calcein (Sigma-Aldrich). The absorbance was measured using the VICTOR™ Multilabel Plate Reader (PerkinElmer, Waltham, MA) at 495 nm.
Scratch test
SH-SY5YCTRL and SH-SY5YLIN28B cells (3 × 104) were plated within each of the two-cell culture reservoirs separated by a 500-μm-thick silicone wall (IBIDI, Milano, Italy). The day after, the silicone insert was removed and the cells were allowed to grow for another 48–72 h. Images were taken every 24 h by a Nikon Eclipse TS100 microscope (Nikon Eclipse TS100, Southern Micro Instruments, Marietta, GA) equipped with a Nikon Coolpix camera. Wound healing was analyzed by Fiji software for each time point.
RNA analysis
Total RNA was extracted from the pool of 30 embryos or cell pellets using TRIzol Reagent (Invitrogen), and 2 μg of RNA were used for cDNA synthesis using Super Script II (Invitrogen), according to the manufacturer’s recommendations. The cDNA was subjected to PCR reaction using the AmpliTaq DNA polymerase (Thermo Fisher Scientific).
The expression of lin28b, cdh1, snail1a, twist1a, and vimentin transcripts was analyzed by real-time quantitative PCR (qPCR) using the SYBR Green PCR Master Mix (Applied Biosystems) in an Applied Biosystems 7900HT Fast Real-Time PCR System. Primer dissociation curves were checked in each run to ensure primer specificity in human and zebrafish mRNA. The expression of gapdh was used as a normalizer in each sample, and triplicate PCR reactions were carried out. Primers were designed using the Primer 3 software and are listed in Supplementary Table S2.
MicroRNA (miRNA) measurement by qPCR
Total RNA was isolated from SH-SY5YCTRL and SH-SY5YLIN28B as well as from the pool of 30 embryos using the Qiazol Lysis Reagent (Qiagen), and 10 ng of total RNA solution were reverse transcribed using the miScript II RT Kit (Qiagen). Specific miRNA levels were quantified by qPCR using the TAQMAN microRNA Assay (Life Technologies) for hsa-miR-let-7a together with the universal RT primer, according to the manufacturer’s protocol. The relative quantities of the miRNAs were calculated using the Cq value after normalization to control the miRNA (RUN6B).
Quantification and statistical analysis
All data were expressed as the mean ± SEM of experimental triplicates. Statistical significance was determined using the Student’s t-test; p < 0.05 was considered significant and was marked with an asterisk (*) on the graphs while p < 0.01 was marked with two asterisks (**).
The statistical significance of the cells’ migratory differences related to time-lapse experiments was calculated using the Wilcoxon test (from the R software ‘stats’ package), and box plots (using the R software “stats” package) reported the quartile distribution of related variables.
Change history
18 November 2019
A Correction to this paper has been published: https://doi.org/10.1038/s41418-019-0451-1
References
Kerosuo L, Bronner-Fraser M. What is bad in cancer is good in the embryo: importance of EMT in neural crest development. Semin Cell Dev Biol. 2012;3:320–32.
Anderson DJ, Carnahan JF, Michelsohn A, Patterson PH. Antibody markers identify a common progenitor to sympathetic neurons and chromaffin cells in vivo and reveal the timing of commitment to neuronal differentiation in the sympathoadrenal lineage. J Neurosci. 1991;11:3507–19.
Kulesa PM, Gammill LS. Neural crest migration: patterns, phases and signals. Dev Biol. 2010;2:566–8.
King CE, Wang L, Winograd R, Madison BB, Mongroo PS, Johnstone CN, et al. LIN28B fosters colon cancer migration, invasion and transformation through let-7-dependent and-independent mechanisms. Oncogene. 2011;30:4185–93.
Xiong H, Zhao W, Wang J, Seifer BJ, Ye C, Chen Y, et al. Oncogenic mechanisms of Lin28 in breast cancer: new functions and therapeutic opportunities. Oncotarget. 2017;8:25721–35.
Hamano R, Miyata H, Yamasaki M, Sugimura K, Tanaka K, Kurokawa Y, et al. High expression of Lin28 is associated with tumour aggressiveness and poor prognosis of patients in oesophagus cancer. Br J Cancer. 2012;106:1415–23.
Molenaar JJ, Domingo-Fernández R, Ebus ME, Lindner S, Koster J, Drabek K, et al. LIN28B induces NB and enhances MYCN levels via let-7 suppression. Nat Genet. 2012;44:1199–206.
Luksch R, Castellani MR, Collini P, De Bernardi B, Conte M, Gambini C, et al. NB (Peripheral neuroblastic tumours). Crit Rev Oncol Hematol. 2016;107:163–81.
De Preter K, Vandesompele J, Heimann P, Yigit N, Beckman S, Schramm A, et al. Human fetal neuroblast and NB transcriptome analysis confirms neuroblast origin and highlights NB candidate genes. Genome Biol. 2006;7:R84.
Shimada H, Ambros IM, Dehner LP, Hata JI, Joshi VV, Roald B, et al. The International NB Pathology Classification (the Shimada system). Cancer. 1999;86:364–72.
Cohn SL, Pearson ADJ, London WB, Monclair T, Ambros PF, Brodeur GM, et al. The International NB Risk Group (INRG) classification system: an INRG task force report. J Clin Oncol. 2009;27:289–97.
Pugh TJ, Morozova O, Attiyeh EF, Asgharzadeh S, Wei JS, Auclair D, et al. The genetic landscape of high-risk NB. Nat Genet. 2013;45:279–84.
Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88:637–46.
Tsialikas J, Romer-Seibert J. LIN28: roles and regulation in development and beyond. Development. 2015;142:2397–404.
Zhou J, Ng SB, Chng WJ. LIN28/LIN28B: an emerging oncogenic driver in cancer stem cells. Int J Biochem Cell Biol. 2013;5:973–8.
Helsmoortel HH, Bresolin S, Lammens T, Cavé H, Noellke P, Caye A, et al. LIN28B overexpression defines a novel fetal-like subgroup of juvenile myelomonocytic leukemia. Blood. 2016;127:1163–72.
Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–76.
Naef V, Monticelli S, Corsinovi D, Mazzetto MT, Cellerino A, Ori M. The age-regulated zinc finger factor ZNF367 is a new modulator of neuroblast proliferation during embryonic neurogenesis. Sci Rep. 2018;8:11836.
Stewart RA, Look AT, Kanki JP, Henion PD. Development of the peripheral sympathetic nervous system in zebrafish. Methods Cell Biol. 2004;76:237–60.
Teitelman G, Baker H, Joh TH, Reis DJ. Appearance of catecholamine-synthesizing enzymes during development of rat sympathetic nervous system: Possible role of tissue environment. Proc Natl Acad Sci USA. 1979;76:509–13.
Dutton JR, Antonellis A, Carney TJ, Rodrigues FSLM, Pavan WJ, Ward A, et al. An evolutionarily conserved intronic region controls the spatiotemporal expression of the transcription factor Sox10. BMC Dev Biol. 2008;8:105.
Luo R, An M, Arduini BL, Henion PD. Specific pan-neural crest expression of zebrafish crestin throughout embryonic development. Dev Dyn. 2001;220:169–74.
Richards M. The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells. 2004;22:51–64.
Kelsh RN, Brand M, Jiang YJ, Heisenberg CP, Lin S, Haffter P, et al. Zebrafish pigmentation mutations and the processes of neural crest development. Development. 1996;123:369–89.
Theveneau E, Mayor R. Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev Biol. 2012;336:34–54.
Heanue TA, Shepherd IT, Burns AJ. Enteric nervous system development in avian and zebrafish models. Dev Biol. 2016;417:129–38.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20.
Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;3:371–79.
Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100.
Kragtorp KA, Miller JR. Integrin alpha5 is required for somite rotation and boundary formation in Xenopus. Dev Dyn. 2007;236:2713–20.
Julich D, Cobb G, Melo AM, McMillen P, Lawton AK, Mochrie SG, et al. Cross-scale integrin regulation organizes ECM and tissue topology. Dev Cell. 2015;34:33–44.
Banning A, Babuke T, Kurrle N, Meister M, Ruonala M, Tikkanen R. Flotillins regulate focal adhesions by interacting with a-actinin and by influencing the activation of focal adhesion kinase. Cells. 2018;7:E28.
Owens DW, McLean GW, Wyke AW, Paraskeva C, Parkinson EK, Frame MC, et al. The catalytic activity of the Src family kinases is required to disrupt cadherin-dependent cell–cell contacts. Mol Biol Cell. 2000;11:51–64.
Avizienyte E, Fincham VJ, Brunton VG, Frame MG. Src SH3/2 domain-mediated peripheral accumulation of Src and phospho-myosin is linked to deregulation of E-cadherin and the epithelial–mesenchymal transition. Mol Biol Cell. 2004;15:2794–803.
Wang H, Zhao Q, Deng K, Guo X, Xia J. Lin28: an emerging important oncogene connecting several aspects of cancer. Tumour Biol. 2016;37:2841–8.
Permuth-Wey J, Kim D, Tsai YY, Lin HY, Chen YA, Barnholtz-Sloan J, et al. LIN28B polymorphisms influence susceptibility to epithelial ovarian cancer. Cancer Res. 2011;71:3896–903.
Nguyen LH, Robinton DA, Seligson MT, Wu L, Li L, Rakheja D, et al. LIN28B is sufficient to drive liver cancer and necessary for its maintenance in murine models. Cancer Cell. 2014;26:248–61.
Zhu S, Lee JS, Guo F, Shin J, Perez-Atayde AR, Kutok JL, et al. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell. 2012;21:362–73.
Hennchen M, Stubbusch J, Abarchan-El Makhfi I, Kramer M, Deller T, Pierre-Eugene C, et al. LIN28B and Let-7 in the control of sympathetic neurogenesis and NB development. J Neurosci. 2015;35:16531–44.
Kalappurakkal JM, Anilkumarr AA, Patra C, van Zanten TS, Sheetz MP, Mayor S. Integrin mechano-chemical signaling generates plasma membrane nanodomains that promote cell spreading. Cell. 2019;177:1738–56.
Laudato S, Patil N, Abba ML, Leupold JH, Benner A, Gaiser T, et al. P53-induced miR-30e-5p inhibits colorectal cancer invasion and metastasis by targeting ITGA6 and ITGB1. Int J Cancer. 2017;141:1879–90.
Brooks DLP, Schwab LP, Krutilina R, Parke DN, Sethuraman A, Hoogewijs D, et al. ITGA6 is directly regulated by hypoxia-inducible factors and enriches for cancer stem cell activity and invasion in metastatic breast cancer models. Mol Cancer. 2016;15:26.
Zhu S, Zhang X, Weichert-Leahey N, Dong Z, Zhang C, Lopez G, et al. LMO1 synergizes with MYCN to promote NB initiation and metastasis. Cancer Cell. 2017;32:310–23.
Kim TH, Kim HI, Soung YH, Shaw LA, Chung J. Integrin (alpha6beta4) signals through Src to increase expression of S100A4, a metastasis promoting factor: implications for cancer cell invasion. Mol Cancer Res. 2009;7:1605–12.
Avizienyte E, Frame MC. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol. 2005;17:542–7.
Crawford BD, Henry CA, Clason TA, Becker AL, Hille MB. Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis. Mol Biol Cell. 2003;14:3065–81.
Bilozur ME, Hay ED. Neural crest migration in 3D extracellular matrix utilizes laminin, fibronectin, or collagen. Dev Biol. 1988;125:19–33.
Westerfield M. The zebrafish book. a guide for the laboratory use of zebrafish (Danio rerio). 3rd ed. Eugene, OR: Univ Oregon Press; 1995.
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310.
Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, et al. The Tol2kit: a multisite gateway-based construction Kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–99.
Holzschuh J, Barrallo-Gimeno A, Ettl AK, Durr K, Knapik EW, Driever W. Noradrenergic neurons in the zebrafish hindbrain are induced by retinoic acid and require tfap2a for expression of the neurotransmitter phenotype. Development. 2003;130:5741–54.
Allende ML, Weinberg ES. The expression pattern of two zebrafish achaete-scute homolog (ash) genes is altered in the embryonic brain of the cyclops mutant. Dev Biol. 1994;166:509–30.
Etard C, Gradl D, Kunz M, Eilers M, Wedlich D. Pontin and Reptin regulate cell proliferation in early Xenopus embryos in collaboration with c-Myc and Miz-1. Mech Dev. 2005;122:545–56.
Giannetti K, Corsinovi D, Rossino C, Appolloni I, Malatesta P, Ori M. Platelet derived growth factor B gene expression in the Xenopus laevis developing central nervous system. Int J Dev Biol. 2016;60:175–9.
Thisse B, Thisse C. In situ hybridization on whole-mount zebrafish embryos and young larvae. Methods Mol Biol. 2014;1211:53–67.
Corallo D, Schiavinato A, Trapani V, Moro E, Argenton F, Bonaldo P. Emilin3 is required for notochord sheath integrity and interacts with Scube2 to regulate notochord-derived Hedgehog signals. Development. 2013;140:4594–601.
Aveic S, Corallo D, Porcù E, Pantile M, Boso D, Zanon C, et al. TP-0903 inhibits neuroblastoma cell growth and enhances the sensitivity to conventional chemotherapy. Eur J Pharm. 2018;818:435–48.
Acknowledgements
The authors would like to thank the Fondazione Italiana per la Lotta al Neuroblastoma and the Fondazione Veronesi (FUV) for their support. We also thank Elisa Lidron (University of Padua), Valentina Tonelotto (University of Padua), and Claudia Grigoletto (University of Trieste) for their excellent technical assistance.
Funding
This work was supported by funds from Fondazione Italiana per la Lotta al Neuroblastoma and from the Fondazione Istituto di Ricerca Pediatrica Città della Speranza (project number 19_07IRP). Corallo Diana was supported by a Fondazione Veronesi (FUV) Postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by E. Baehrecke
Supplementary information
Rights and permissions
About this article
Cite this article
Corallo, D., Donadon, M., Pantile, M. et al. LIN28B increases neural crest cell migration and leads to transformation of trunk sympathoadrenal precursors. Cell Death Differ 27, 1225–1242 (2020). https://doi.org/10.1038/s41418-019-0425-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-019-0425-3
This article is cited by
-
Genetic predisposition and chromosome instability in neuroblastoma
Cancer and Metastasis Reviews (2020)