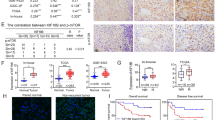
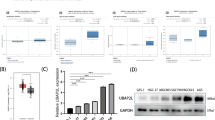
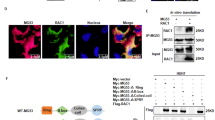
Abstract
A20 acts as a tumor suppressor in hepatocellular carcinoma, especially inhibiting metastasis of the malignant cells. However, the mechanisms whereby A20 plays the inhibitory roles are not understood completely. Rac1 signaling is essential for cell migration in hepatocellular carcinoma metastasis. Nevertheless, it is not known whether and how A20 inhibits Rac1 signaling to suppress the migration of hepatocellular carcinoma cell. Thereby, we analyzed the relationship between A20 and Rac1 activation, as well as the activity of Akt and mTORC2, two signaling components upstream of Rac1, using gain and loss of function experiments. We found that the overexpression of A20 repressed, while the knockdown or knockout of A20 promoted, the activation of Rac1, Akt and mTORC2 in hepatocellular carcinoma cells. Moreover, the inhibitory effect of A20 on the mTORC2/Akt/Rac1 signaling axis was due to the interaction between A20 and mTORC2 complex. The binding of A20 to mTORC2 was mediated by the ZnF7 domain of A20 and M1 ubiquitin chain in the mTORC2 complex. Furthermore, A20 inhibited metastasis of hepatocellular carcinoma cells via restraining mTORC2 in a hepatocellular carcinoma xenograft mouse model. These findings revealed the relationship between A20 and mTORC2, and explained the molecular mechanisms of A20 in inhibition of hepatocellular carcinoma metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Catrysse L, Vereecke L, Beyaert R, van Loo G. A20 in inflammation and autoimmunity. Trends Immunol. 2014;35:22–31.
Hymowitz SG, Wertz IE. A20: from ubiquitin editing to tumour suppression. Nat Rev Cancer. 2010;10:332–41.
Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 2004;430:694–9.
Li L, Soetandyo N, Wang Q, Ye Y. The zinc finger protein A20 targets TRAF2 to the lysosomes for degradation. Biochim Biophys Acta. 2009;1793:346–53.
Garg AV, Ahmed M, Vallejo AN, Ma A, Gaffen SL. The deubiquitinase A20 mediates feedback inhibition of interleukin-17 receptor signaling. Sci Signal. 2013;6:ra44.
Lu TT, Onizawa M, Hammer GE, Turer EE, Yin Q, Damko E, et al. Dimerization and ubiquitin mediated recruitment of A20, a complex deubiquitinating enzyme. Immunity 2013;38:896–905.
Verhelst K, Carpentier I, Kreike M, Meloni L, Verstrepen L, Kensche T, et al. A20 inhibits LUBAC-mediated NF-kappaB activation by binding linear polyubiquitin chains via its zinc finger 7. The. EMBO J. 2012;31:3845–55.
Shi Y, Wang X, Wang J, Wang X, Zhou H, Zhang L. The dual roles of A20 in cancer. Cancer Lett. 2021;511:26–35.
Catrysse L, Farhang Ghahremani M, Vereecke L, Youssef SA, Mc Guire C, Sze M, et al. A20 prevents chronic liver inflammation and cancer by protecting hepatocytes from death. Cell death Dis. 2016;7:e2250.
Chen H, Hu L, Luo Z, Zhang J, Zhang C, Qiu B, et al. A20 suppresses hepatocellular carcinoma proliferation and metastasis through inhibition of Twist1 expression. Mol Cancer. 2015;14:186.
Wang X, Ma C, Zong Z, Xiao Y, Li N, Guo C, et al. A20 inhibits the motility of HCC cells induced by TNF-alpha. Oncotarget 2016;7:14742–54.
Qin G, Luo M, Chen J, Dang Y, Chen G, Li L, et al. Reciprocal activation between MMP-8 and TGF-beta1 stimulates EMT and malignant progression of hepatocellular carcinoma. Cancer Lett. 2016;374:85–95.
Huang L, Fu L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B 2015;5:390–401.
Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–65.
Cuesta C, Arevalo-Alameda C, Castellano E. The importance of being PI3K in the RAS signaling network. Genes (Basel). 2021;12:1094.
Revathidevi S, Munirajan AK. Akt in cancer: mediator and more. Semin Cancer Biol. 2019;59:80–91.
Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019;79:1019–31.
Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20:74–88.
Brown JS, Banerji U. Maximising the potential of AKT inhibitors as anti-cancer treatments. Pharm Ther. 2017;172:101–15.
Toulany M, Rodemann HP. Phosphatidylinositol 3-kinase/Akt signaling as a key mediator of tumor cell responsiveness to radiation. Semin Cancer Biol. 2015;35:180–90.
Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–8.
Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–56.
Yuan T, Lupse B, Maedler K, Ardestani A. mTORC2 signaling: a path for pancreatic beta cell’s growth and function. J Mol Biol. 2018;430:904–18.
Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–71.
Zhu G, Fan Z, Ding M, Zhang H, Mu L, Ding Y, et al. An EGFR/PI3K/AKT axis promotes accumulation of the Rac1-GEF Tiam1 that is critical in EGFR-driven tumorigenesis. Oncogene 2015;34:5971–82.
Morrison Joly M, Williams MM, Hicks DJ, Jones B, Sanchez V, Young CD, et al. Two distinct mTORC2-dependent pathways converge on Rac1 to drive breast cancer metastasis. Breast Cancer Res. 2017;19:74.
Bailly C, Beignet J, Loirand G, Sauzeau V. Rac1 as a therapeutic anticancer target: Promises and limitations. Biochem Pharm. 2022;203:115180.
Bagrodia S, Cerione RA. PAK to the future. Trends Cell Biol. 1999;9:350–5.
Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009;28:51–63.
George A, Martin GB. Frank McCormick and Arie Abol. A novel serine kinase activated by rac1 cdc42hs-dependent autophosphorylation is related to pak65 and ste20. The. EMBO J. 1995;14:1970–8.
Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4:a011189.
Henderson V, Smith B, Burton LJ, Randle D, Morris M, Odero-Marah VA. Snail promotes cell migration through PI3K/AKT-dependent Rac1 activation as well as PI3K/AKT-independent pathways during prostate cancer progression. Cell Adh Migr. 2015;9:255–64.
Bhat AV, Palanichamy Kala M, Rao VK, Pignata L, Lim HJ, Suriyamurthy S, et al. Epigenetic regulation of the PTEN-AKT-RAC1 axis by G9a is critical for tumor growth in alveolar rhabdomyosarcoma. Cancer Res. 2019;79:2232–43.
Jiang Y, Su S, Zhang Y, Qian J, Liu P. Control of mTOR signaling by ubiquitin. Oncogene 2019;38:3989–4001.
Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302.
Kirisako T, Kamei K, Murata S, Kato M, Fukumoto H, Kanie M, et al. A ubiquitin ligase complex assembles linear polyubiquitin chains. EMBO J. 2006;25:4877–87.
Tokunaga F, Iwai K. LUBAC, a novel ubiquitin ligase for linear ubiquitination, is crucial for inflammation and immune responses. Microbes Infect. 2012;14:563–72.
Feng Y, Zhang Y, Cai Y, Liu R, Lu M, Li T, et al. A20 targets PFKL and glycolysis to inhibit the progression of hepatocellular carcinoma. Cell Death Dis. 2020;11:89.
Zhang SQ, Kovalenko A, Cantarella G, Wallach D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKgamma) upon receptor stimulation. Immunity 2000;12:301–11.
Lork M, Verhelst K, Beyaert RCYLD. A20 and OTULIN deubiquitinases in NF-kappaB signaling and cell death: so similar, yet so different. Cell Death Differ. 2017;24:1172–83.
Draber P, Kupka S, Reichert M, Draberova H, Lafont E, de Miguel D, et al. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Rep. 2015;13:2258–72.
Yamaguchi N, Yamaguchi N. The seventh zinc finger motif of A20 is required for the suppression of TNF-alpha-induced apoptosis. FEBS Lett. 2015;589:1369–75.
Onizawa M, Oshima S, Schulze-Topphoff U, Oses-Prieto JA, Lu T, Tavares R, et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat Immunol. 2015;16:618–27.
Zhang P, Wang PX, Zhao LP, Zhang X, Ji YX, Zhang XJ, et al. The deubiquitinating enzyme TNFAIP3 mediates inactivation of hepatic ASK1 and ameliorates nonalcoholic steatohepatitis. Nat Med. 2018;24:84–94.
Lv Q, Xie L, Cheng Y, Shi Y, Shan W, Ning C, et al. A20-mediated deubiquitination of ERalpha in the microenvironment of CD163(+) macrophages sensitizes endometrial cancer cells to estrogen. Cancer Lett. 2019;442:137–47.
Slowicka K, Serramito-Gomez I, Boada-Romero E, Martens A, Sze M, Petta I, et al. Physical and functional interaction between A20 and ATG16L1-WD40 domain in the control of intestinal homeostasis. Nat Commun. 2019;10:1834.
Zhai Y, Lin P, Feng Z, Lu H, Han Q, Chen J, et al. TNFAIP3-DEPTOR complex regulates inflammasome secretion through autophagy in ankylosing spondylitis monocytes. Autophagy 2018;14:1629–43.
Matsuzawa Y, Oshima S, Takahara M, Maeyashiki C, Nemoto Y, Kobayashi M, et al. TNFAIP3 promotes survival of CD4 T cells by restricting MTOR and promoting autophagy. Autophagy 2015;11:1052–62.
Yuan W, Chen Y, Zhou Y, Bao K, Yu X, Xu Y, et al. Formononetin attenuates atopic dermatitis by upregulating A20 expression via activation of G protein-coupled estrogen receptor. J Ethnopharmacol. 2021;266:113397.
Jia F, Deng F, Xu P, Li S, Wang X, Hu P, et al. NOD1 agonist protects against lipopolysaccharide and D-galactosamine-induced fatal hepatitis through the upregulation of A20 expression in hepatocytes. Front Immunol. 2021;12:603192.
Wang Y, Park NY, Jang Y, Ma A, Jiang Q. Vitamin E gamma-tocotrienol inhibits cytokine-stimulated NF-kappaB activation by induction of anti-inflammatory A20 via stress adaptive response due to modulation of sphingolipids. J Immunol. 2015;195:126–33.
Davies S, Dai D, Feldman I, Pickett G, Leslie KK. Identification of a novel mechanism of NF-kappaB inactivation by progesterone through progesterone receptors in Hec50co poorly differentiated endometrial cancer cells: induction of A20 and ABIN-2. Gynecologic Oncol. 2004;94:463–70.
Chen CJ, Sgritta M, Mays J, Zhou H, Lucero R, Park J, et al. Therapeutic inhibition of mTORC2 rescues the behavioral and neurophysiological abnormalities associated with Pten-deficiency. Nat Med. 2019;25:1684–90.
Masui K, Harachi M, Cavenee WK, Mischel PS, Shibata N. mTOR complex 2 is an integrator of cancer metabolism and epigenetics. Cancer Lett. 2020;478:1–7.
Mossmann D, Park S, Hall MN. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer. 2018;18:744–57.
Acknowledgements
This work was supported by the National Nature Science Foundation of China [grant numbers 81672806 and 81972685] and the Key Research and Development Program of Shandong Province [grant number 2017GSF218066].
Author information
Authors and Affiliations
Contributions
XW designed and carried out most of the experiments, and wrote the original draft. YX and ZW contributed to cell culture. YD and JY performed in vivo experiments. JW and XW contributed to data analysis. HZ and LZ contributed to interpretation of the results and writing-reviewing. YS conceived the study, supervised the research and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
All procedures for animal experiments were approved by the Animal Care and Utilization Committee of Shandong University and performed in a manner compliant with all relevant ethical regulations regarding animal research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Xiao, Y., Dong, Y. et al. A20 interacts with mTORC2 to inhibit the mTORC2/Akt/Rac1 signaling axis in hepatocellular carcinoma cells. Cancer Gene Ther 30, 424–436 (2023). https://doi.org/10.1038/s41417-022-00562-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-022-00562-2