Abstract
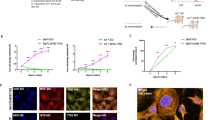
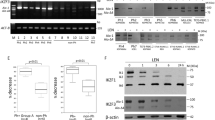
JAK2 rearrangements can occur in Philadelphia chromosome-like acute lymphoblastic leukemia (Ph-like ALL). Here, we performed functional analysis of the SPAG9::JAK2 fusion, which was identified in a pediatric patient with Ph-like ALL, to establish molecular targeted therapy. Ba/F3 cells expressing SPAG9::JAK2 generated by retroviral transduction (Ba/F3-SPAG9-JAK2), proliferated in the absence of IL-3, and exhibited constitutive phosphorylation of the tyrosine residues in the JAK2 kinase domain of the fusion protein and STAT3/STAT5. Mutation of tyrosine residues in the JAK2 kinase domain (SPAG9::JAK2 mut) abolished IL-3 independence, but had no influence on STAT3/STAT5 phosphorylation levels. Gene expression analysis revealed that Stat1 was significantly upregulated in Ba/F3-SPAG9-JAK2 cells. STAT1 was also phosphorylated in Ba/F3-SPAG9-JAK2 but not SPAG9-JAK2 mut cells, suggesting that STAT1 is key for SPAG9::JAK2-mediated cell proliferation. Consistently, STAT1 induced expression of the anti-apoptotic proteins, BCL-2 and MCL-1, as did SPAG9::JAK2, but not SPAG9::JAK2 mut. Ruxolitinib abrogated Ba/F3-SPAG9-JAK2-mediated proliferation in vitro, but was insufficient in vivo. Venetoclax (a BCL-2 inhibitor) or AZD5991 (an MCL-1 inhibitor) enhanced the effects of ruxolitinib on Ba/F3-SPAG9-JAK2 in vitro. These findings suggest that activation of the JAK2-STAT1-BCL-2/MCL-1 axis contributes to SPAG9::JAK2-related aberrant growth promotion. BCL-2 or MCL-1 inhibition is a potential therapeutic option for B-ALL with SPAG9::JAK2 fusion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Tasian SK, Loh ML, Hunger SP. Philadelphia chromosome-like acute lymphoblastic leukemia. Blood. 2017;130:2064–72.
Roberts KG, Gu Z, Payne-Turner D, McCastlain K, Harvey RC, Chen IM, et al. High frequency and poor outcome of philadelphia chromosome-like acute lymphoblastic leukemia in adults. J Clin Oncol. 2017;35:394–401.
Kawamura M, Taki T, Kaku H, Ohki K, Hayashi Y. Identification of SPAG9 as a novel JAK2 fusion partner gene in pediatric acute lymphoblastic leukemia with t(9;17)(p24;q21). Genes Chromosomes Cancer. 2015;54:401–8.
Reshmi SC, Harvey RC, Roberts KG, Stonerock E, Smith A, Jenkins H, et al. Targetable kinase gene fusions in high-risk B-ALL: a study from the Children’s Oncology Group. Blood. 2017;129:3352–61.
Schinnerl D, Fortschegger K, Kauer M, Marchante JR, Kofler R, Den Boer ML, et al. The role of the Janus-faced transcription factor PAX5-JAK2 in acute lymphoblastic leukemia. Blood. 2015;125:1282–91.
Schwaller J, Parganas E, Wang D, Cain D, Aster JC, Williams IR, et al. Stat5 is essential for the myelo- and lymphoproliferative disease induced by TEL/JAK2. Mol Cell. 2000;6:693–704.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Waibel M, Solomon VS, Knight DA, Ralli RA, Kim SK, Banks KM, et al. Combined targeting of JAK2 and Bcl-2/Bcl-xL to cure mutant JAK2-driven malignancies and overcome acquired resistance to JAK2 inhibitors. Cell Rep. 2013;5:1047–59.
Prutsch N, Gurnhofer E, Suske T, Liang HC, Schlederer M, Roos S, et al. Dependency on the TYK2/STAT1/MCL1 axis in anaplastic large cell lymphoma. Leukemia. 2019;33:696–709.
Hsu WL, Chiu TH, Tai DJ, Ma YL, Lee EH. A novel defense mechanism that is activated on amyloid-beta insult to mediate cell survival: role of SGK1-STAT1/STAT2 signaling. Cell Death Differ. 2009;16:1515–29.
Timofeeva OA, Plisov S, Evseev AA, Peng S, Jose-Kampfner M, Lovvorn HN, et al. Serine-phosphorylated STAT1 is a prosurvival factor in Wilms’ tumor pathogenesis. Oncogene. 2006;25:7555–64.
Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–6.
Tomii T, Imamura T, Tanaka K, Kato I, Mayumi A, Soma E, et al. Leukemic cells expressing NCOR1-LYN are sensitive to dasatinib in vivo in a patient-derived xenograft mouse model. Leukemia 2020;35:2092–6.
Ho JM, Beattie BK, Squire JA, Frank DA, Barber DL. Fusion of the ets transcription factor TEL to Jak2 results in constitutive Jak-Stat signaling. Blood. 1999;93:4354–64.
Dorritie KA, McCubrey JA, Johnson DE. STAT transcription factors in hematopoiesis and leukemogenesis: opportunities for therapeutic intervention. Leukemia. 2014;28:248–57.
Yu H, Jove R. The STATs of cancer-new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105.
Sanda T, Tyner JW, Gutierrez A, Ngo VN, Glover J, Chang BH, et al. TYK2-STAT1-BCL2 pathway dependence in T-cell acute lymphoblastic leukemia. Cancer Disco. 2013;3:564–77.
Kulling PM, Olson KC, Hamele CE, Toro MF, Tan SF, Feith DJ, et al. Dysregulation of the IFN-gamma-STAT1 signaling pathway in a cell line model of large granular lymphocyte leukemia. PLoS One. 2018;13:e0193429.
Kawashima-Goto S, Imamura T, Tomoyasu C, Yano M, Yoshida H, Fujiki A, et al. BCL2 inhibitor (ABT-737): a restorer of prednisolone sensitivity in early T-cell precursor-acute lymphoblastic leukemia with high MEF2C expression? PLoS One. 2015;10:e0132926.
Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–6.
Doshi KA, Trotta R, Natarajan K, Rassool FV, Tron AE, Huszar D, et al. Pim kinase inhibition sensitizes FLT3-ITD acute myeloid leukemia cells to topoisomerase 2 inhibitors through increased DNA damage and oxidative stress. Oncotarget. 2016;7:48280–95.
Yoshida H, Imamura T, Fujiki A, Hirashima Y, Miyachi M, Inukai T, et al. Post-transcriptional modulation of C/EBPalpha prompts monocytic differentiation and apoptosis in acute myelomonocytic leukaemia cells. Leuk Res. 2012;36:735–41.
Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, et al. NOD/SCID/γcnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–82.
Kato I, Nishinaka Y, Nakamura M, Akarca AU, Niwa A, Ozawa H, et al. Hypoxic adaptation of leukemic cells infiltrating the CNS affords a therapeutic strategy targeting VEGFA. Blood. 2017;129:3126–9.
Acknowledgements
This study was supported by grants-in-aid for scientific research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (17K10124), and by a Grant-in-Aid for Practical Research for Innovative Cancer Control (17ck0106253h0001, 18ck0106253h0002, and 19ck0106253h0003).
Author information
Authors and Affiliations
Contributions
AM designed the research, performed the experiments, analyzed the data, generated the figures, and wrote the manuscript. TI designed the research, analyzed the data, and wrote the manuscript. TT, TK, TM, KT, and HU performed the experiments and analyzed the data. HY, IK, MK, TN, JT, and HH supervised the research and participated in editing the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mayumi, A., Tomii, T., Kanayama, T. et al. The combination of ruxolitinib and Bcl-2/Mcl-1 inhibitors has a synergistic effect on leukemic cells carrying a SPAG9::JAK2 fusion. Cancer Gene Ther 29, 1930–1938 (2022). https://doi.org/10.1038/s41417-022-00511-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-022-00511-z
This article is cited by
-
JAK/STAT in leukemia: a clinical update
Molecular Cancer (2024)