Abstract
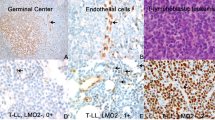
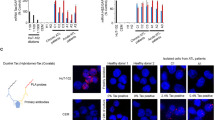
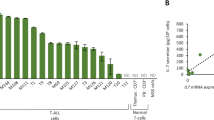
A massive increase in the number of mature CD4+ T-cells in peripheral blood (PB) is a defining characteristic of acute type of adult T-cell leukemia (ATL). To date, the site of proliferation of ATL cells in the body has been unclear. In an attempt to address this question, we examined the expression of the proliferation marker, Ki-67, in freshly isolated ATL cells from PB and lymph nodes (LNs) of patients with various types of ATL. Our findings reveal that LN-ATL cells display higher expression of the Ki-67 antigen than PB-ATL cells in acute type patients. The gene expression of T-cell quiescence regulators such as Krüppel-like factor 2/6 and forkhead box protein 1 was substantially high in acute type PB-ATL cells. The expression of human telomerase reverse transcriptase, which is involved in T-cell expansion, was significantly low in PB-ATL cells from acute type patients, similar to that in normal resting T-cells. These findings suggest that ATL cells proliferate in the LNs rather than in PB.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Not applicable.
References
Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–9.
Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita KI, et al. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–80.
Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–92.
Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87). Br J Haematol. 1991;79:428–37.
Tsukasaki K, Utsunomiya A, Fukuda H, Shibata T, Fukushima T, Takatsuka Y, et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol. 2007;25:5458–64.
Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837–42.
Utsunomiya A, Choi I, Chihara D, Seto M. Recent advances in the treatment of adult T-cell leukemia-lymphomas. Cancer Sci. 2015;106:344–51.
Shirono K, Hattori T, Hata H, Nishimura H, Takatsuki K. Profiles of expression of activated cell antigens on peripheral blood and lymph node cells from different clinical stages of adult T-cell leukemia. Blood. 1989;73:1664–71.
Yamada Y, Murata K, Kamihira S, Atogami S, Tsukasaki K, Sohda H, et al. Prognostic significance of the proportion of Ki-67-positive cells in adult T-cell leukemia. Cancer. 1991;67:2605–9.
Katsuya H, Ishitsuka K, Utsunomiya A, Hanada S, Eto T, Moriuchi Y, et al. Treatment and survival among 1594 patients with ATL. Blood. 2015;126:2570–7.
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–5.
Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, et al. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770–1.
Sugamura K, Fujii M, Kannagi M, Sakitani M, Takeuchi M, Hinuma Y. Cell surface phenotypes and expression of viral antigens of various human cell lines carrying human T-cell leukemia virus. Int J Cancer. 1984;34:221–8.
Hori T, Uchiyama T, Tsudo M, Umadome H, Ohno H, Fukuhara S, et al. Establishment of an interleukin 2-dependent human T cell line from a patient with T cell chronic lymphocytic leukemia who is not infected with human T cell leukemia/lymphoma virus. Blood. 1987;70:1069–72.
Yamada Y, Nagata Y, Kamihira S, Tagawa M, Ichimaru M, Tomonaga M, et al. IL-2-dependent ATL cell lines with phenotypes differing from the original leukemia cells. Leuk Res. 1991;15:619–25.
Maeda T, Yamada Y, Moriuchi R, Sugahara K, Tsuruda K, Joh T, et al. Fas gene mutation in the progression of adult T cell leukemia. J Exp Med. 1999;189:1063–71.
Yamada Y, Sugahara K, Tsuruda K, Nohda K, Hata T, Maeda T, et al. Fas-resistance in ATL cell lines not associated with HTLV-I or FAP-1 production. Cancer Lett. 1999;147:215–9.
Mizuguchi M, Hara T, Yoshita-Takahashi M, Kohda T, Tanaka Y, Nakamura M. Promoter CpG methylation inhibits Krüppel-like factor 2 (KLF2)-Mediated repression of hTERT gene expression in human T-cells. Biochem Biophys Rep. 2021;26:100984.
Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011;12:323.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40.
Shao H, Yuan CM, Xi L, Raffeld M, Morris JC, Janik JE, et al. Minimal residual disease detection by flow cytometry in adult T-cell leukemia/lymphoma. Am J Clin Pathol. 2010;133:592–601.
Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10:176–84.
Feng X, Ippolito GC, Tian L, Wiehagen K, Oh S, Sambandam A, et al. Foxp1 is an essential transcriptional regulator for the generation of quiescent naive T cells during thymocyte development. Blood. 2010;115:510–8.
Cao Z, Sun X, Icli B, Wara AK, Feinberg MW. Role of Krüppel-like factors in leukocyte development, function, and disease. Blood. 2010;116:4404–14.
Hwang SS, Lim J, Yu Z, Kong P, Sefik E, Xu H, et al. mRNA destabilization by BTG1 and BTG2 maintains T cell quiescence. Science. 2020;367:1255–60.
Hara T, Mizuguchi M, Fujii M, Nakamura M. Krüppel-like factor 2 represses transcription of the telomerase catalytic subunit human telomerase reverse transcriptase (hTERT) in human T cells. J Biol Chem. 2015;290:8758–63.
Kuo CT, Veselits ML, Leiden JM. LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science. 1997;277:1986–90.
Buckley AF, Kuo CT, Leiden JM. Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc-dependent pathway. Nat Immunol. 2001;2:698–704.
Zinn RL, Pruitt K, Eguchi S, Baylin SB, Herman JG. hTERT is expressed in cancer cell lines despite promoter DNA methylation by preservation of unmethylated DNA and active chromatin around the transcription start site. Cancer Res. 2007;67:194–201.
Guilleret I, Benhattar J. Unusual distribution of DNA methylation within the hTERT CpG island in tissues and cell lines. Biochem Biophys Res Commun. 2004;325:1037–43.
Guilleret I, Yan P, Grange F, Braunschweig R, Bosman FT, Benhattar J. Hypermethylation of the human telomerase catalytic subunit (hTERT) gene correlates with telomerase activity. Int J Cancer. 2002;101:335–41.
Furuta R, Yasunaga JI, Miura M, Sugata K, Saito A, Akari H, et al. Human T-cell leukemia virus type 1 infects multiple lineage hematopoietic cells in vivo. PLoS Pathog. 2017;13:e1006722.
Watanabe T. Adult T-cell leukemia: molecular basis for clonal expansion and transformation of HTLV-1-infected T cells. Blood. 2017;129:1071–81.
Bittencourt AL, Mota K, Oliveira RF, Farre L. A dyshidrosis-like variant of adult T-cell leukemia/lymphoma with clinicopathological aspects of mycosis fungoides. A case report. Am J Dermatopathol. 2009;31:834–7.
Bittencourt AL, Barbosa HS, Vieira MD, Farre L. Adult T-cell leukemia/lymphoma (ATL) presenting in the skin: clinical, histological and immunohistochemical features of 52 cases. Acta Oncol. 2009;48:598–604.
Prada-Arismendy J, Arroyave JC, Rothlisberger S. Molecular biomarkers in acute myeloid leukemia. Blood Rev. 2017;31:63–76.
Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5:172–83.
Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. New Engl J Med. 2015;373:1541–52.
Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet. 2018;391:1524–37.
Kikushige Y, Ishikawa F, Miyamoto T, Shima T, Urata S, Yoshimoto G, et al. Self-renewing hematopoietic stem cell is the primary target in pathogenesis of human chronic lymphocytic leukemia. Cancer Cell. 2011;20:246–59.
Sasaki H, Nishikata I, Shiraga T, Akamatsu E, Fukami T, Hidaka T, et al. Overexpression of a cell adhesion molecule, TSLC1, as a possible molecular marker for acute-type adult T-cell leukemia. Blood. 2005;105:1204–13.
Nakahata S, Saito Y, Marutsuka K, Hidaka T, Maeda K, Hatakeyama K, et al. Clinical significance of CADM1/TSLC1/IgSF4 expression in adult T-cell leukemia/lymphoma. Leukemia. 2012;26:1238–46.
Acknowledgements
We thank A. Yamashita for critiquing the manuscript, C. Nagamine for providing technical assistance, and all the members of our laboratory for their suggestions and encouragement. This manuscript was supported by Grants-in-Aid for Scientific Research 19K08843 (M.M.) and JP23501258 (N.M.) from the Japan Society for the Promotion of Science and the Project of Establishing Medical Research Base Networks against Infectious Diseases in Okinawa (Y.T. and T.F.).
Author information
Authors and Affiliations
Contributions
MM, MN, and YTan conceived and designed the experiments; MM, MT, SS, MYT, and NI performed the experiments and analyzed the data; YTak, KK, TF, and YTan collected the samples and provided advice; HH provided HTLV-1–infected cell lines; MM, MN, and YTan wrote the manuscript, and all the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mizuguchi, M., Takatori, M., Sakihama, S. et al. Acute type adult T-cell leukemia cells proliferate in the lymph nodes rather than in peripheral blood. Cancer Gene Ther 29, 1570–1577 (2022). https://doi.org/10.1038/s41417-022-00475-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-022-00475-0