Abstract
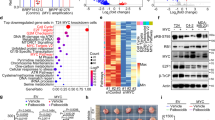
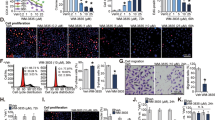
The proto-oncogene cellular myelocytomatosis (c-Myc) is a transcription factor that is upregulated in several human cancers. Therapeutic targeting of c-Myc remains a challenge because of a disordered protein tertiary structure. The basic helical structure and zipper protein of c-Myc forms an obligate heterodimer with its partner MYC-associated factor X (MAX) to function as a transcription factor. An attractive strategy is to inhibit MYC/MAX dimerization to decrease c-Myc transcriptional function. Several methods have been described to inhibit MYC/MAX dimerization including small molecular inhibitors and proteomimetics. We studied the effect of a second-generation small molecular inhibitor 3JC48-3 on prostate cancer growth and viability. In our experimental studies, we found 3JC48-3 decreases prostate cancer cells’ growth and viability in a dose-dependent fashion in vitro. We confirmed inhibition of MYC/MAX dimerization by 3JC48-3 using immunoprecipitation experiments. We have previously shown that the MYC/MAX heterodimer is a transcriptional repressor of a novel kinase protein kinase D1 (PrKD1). Treatment with 3JC48-3 upregulated PrKD1 expression and phosphorylation of known PrKD1 substrates: the threonine 120 (Thr-120) residue in beta-catenin and the serine 216 (Ser-216) in Cell Division Cycle 25 (CDC25C). The mining of gene expression in human metastatic prostate cancer samples demonstrated an inverse correlation between PrKD1 and c-Myc expression. Normal mice and mice with patient-derived prostate cancer xenografts (PDX) tolerated intraperitoneal injections of 3JC48-3 up to 100 mg/kg body weight without dose-limiting toxicity. Preliminary results in these PDX mouse models suggest that 3JC48-3 may be effective in decreasing the rate of tumor growth. In conclusion, our study demonstrates that 3JC48-3 is a potent MYC/MAX heterodimerization inhibitor that decreases prostate cancer growth and viability associated with upregulation of PrKD1 expression and kinase activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data used to generate Fig. 3D can be found at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE8511. All other data generated and analyzed during this study can be found within this article.
References
Zeng W, Sun H, Meng F, Liu Z, Xiong J, Zhou S, et al. Nuclear C-MYC expression level is associated with disease progression and potentially predictive of two year overall survival in prostate cancer. Int J Clin Exp Pathol. 2015;8:1878–88.
Yu C, Niu X, Jin F, Liu Z, Jin C, Lai L. Structure-based Inhibitor Design for the Intrinsically Disordered Protein c-Myc. Sci Rep. 2016;6:22298.
Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000;16:653–99.
Singh SS, Jois SD. Homo- and heterodimerization of proteins in cell signaling: inhibition and drug design. Adv Protein Chem Struct Biol. 2018;111:1–59.
Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan T, et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther. 2021;6:201.
Shin WH, Kumazawa K, Imai K, Hirokawa T, Kihara D. Current challenges and opportunities in designing protein-protein interaction targeted drugs. Adv Appl Bioinform Chem. 2020;13:11–25.
Yap JL, Wang H, Hu A, Chauhan J, Jung KY, Gharavi RB, et al. Pharmacophore identification of c-Myc inhibitor 10074-G5. Bioorg Med Chem Lett. 2013;23:370–4.
Chauhan J, Wang H, Yap JL, Sabato PE, Hu A, Prochownik EV, et al. Discovery of methyl 4′-methyl-5-(7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)-[1,1′-biphenyl]-3-carboxylate, an improved small-molecule inhibitor of c-Myc-max dimerization.Chem Med Chem. 2014;9:2274–2285.
Smith K, Dalton S. Myc transcription factors: key regulators behind establishment and maintenance of pluripotency. Regen Med. 2010;5:947–59.
Nickkholgh B, Sittadjody S, Rothberg MB, Fang X, Li K, Chou JW, et al. Beta-catenin represses protein kinase D1 gene expression by non-canonical pathway through MYC/MAX transcription complex in prostate cancer. Oncotarget 2017;8:78811–24.
Youssef I, Ricort JM. Deciphering the role of protein kinase D1 (PKD1) in cellular proliferation. Mol Cancer Res. 2019;17:1961–74.
Steinberg SF. Regulation of protein kinase D1 activity. Mol Pharmacol. 2012;81:284–91.
Sundram V, Chauhan SC, Jaggi M. Emerging roles of protein kinase D1 in cancer. Mol Cancer Res. 2011;9:985–96.
Du C, Jaggi M, Zhang C, Balaji KC. Protein kinase D1-mediated phosphorylation and subcellular localization of beta-catenin. Cancer Res. 2009;69:1117–24.
Nickkholgh B, Sittadjody S, Ordonez K, Rothberg MB, Balaji KC. Protein kinase D1 induces G1-phase cell-cycle arrest independent of Checkpoint kinases by phosphorylating Cell Division Cycle Phosphatase 25. Prostate 2019;79:1053–8.
Arsura M, Deshpande A, Hann SR, Sonenshein GE. Variant Max protein, derived by alternative splicing, associates with c-Myc in vivo and inhibits transactivation. Mol Cell Biol. 1995;15:6702–9.
Giffney HE, Cummins EP, Murphy EP, Brayden DJ, Crean D. Protein kinase D, ubiquitin and proteasome pathways are involved in adenosine receptor-stimulated NR4A expression in myeloid cells. Biochem Biophys Res Commun. 2021;555:19–25.
Wang S, Wang J, Lv X. Selection of reference genes for expression analysis in mouse models of acute alcoholic liver injury. Int J Mol Med. 2018;41:3527–36.
Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: archive for functional genomics data sets-update. Nucleic Acids Res. 2013;41:D991–5.
Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–10.
Chen C, Lin W, Huang Y, Chen X, Wang H, Teng L. The essential factors of establishing patient-derived tumor model. J Cancer. 2021;12:28–37.
Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013;32:5501–11.
Kiessling A, Sperl B, Hollis A, Eick D, Berg T. Selective inhibition of c-Myc/Max dimerization and DNA binding by small molecules. Chem Biol. 2006;13:745–51.
Jubb H, Blundell TL, Ascher DB. Flexibility and small pockets at protein-protein interfaces: new insights into druggability. Prog Biophys Mol Biol. 2015;119:2–9.
Lu H, Zhou Q, He J, Jiang Z, Peng C, Tong R, et al. Recent advances in the development of protein–protein interactions modulators: mechanisms and clinical trials. Signal Transduct Target Ther. 2020;5:213.
Berg T, Cohen SB, Desharnais J, Sonderegger C, Maslyar DJ, Goldberg J, et al. Small-molecule antagonists of Myc/Max dimerization inhibit Myc-induced transformation of chicken embryo fibroblasts. Proc Natl Acad Sci USA. 2002;99:3830.
Bedard PL, Hyman DM, Davids MS, Siu LL. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet 2020;395:1078–88.
Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull. 2017;7:339–48.
Lv D, Chen H, Feng Y, Cui B, Kang Y, Zhang P, et al. Small-molecule inhibitor targeting protein kinase D: a potential therapeutic strategy. Front Oncol. 2021;11:680221.
Madden SK, de Araujo AD, Gerhardt M, Fairlie DP, Mason JM. Taking the Myc out of cancer: toward therapeutic strategies to directly inhibit c-Myc. Mol Cancer. 2021;20:3.
Acknowledgements
This study was supported by the Department of Defense under Award Number W81XWH-19-1-0745 to K.C.B.
Author information
Authors and Affiliations
Contributions
Conceptualization by S.S., S.F., J.C., T.O., and K.C.B.; Methodology by S.S., S.F., J.C., T.O., and K.C.B.; Validation by S.F., S.K., and K.C.B.; Formal analysis by S.S., J.C., Y.M., P.K.S., and C.R.; Resources by S.F., S.K., and K.C.B.; Data curation by S.S., S.F., Y.M., P.K.S., and K.C.B.; Writing by S.S., V.C., and C.R.; Original draft by S.S., S.F., V.C., and C.R.; Writing – Review and Editing by S.S., V.C., C.R., and K.C.B.; Supervision by S.F., S.K., and K.C.B.; Project administration by K.C.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Florida. All procedures adhered with the most recent specifications of the National Research Council (US) Committee for the Care and Use of Laboratory Animals (2011).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shukla, S., Fletcher, S., Chauhan, J. et al. 3JC48-3 (methyl 4′-methyl-5-(7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)-[1,1′-biphenyl]-3-carboxylate): a novel MYC/MAX dimerization inhibitor reduces prostate cancer growth. Cancer Gene Ther 29, 1550–1557 (2022). https://doi.org/10.1038/s41417-022-00455-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-022-00455-4