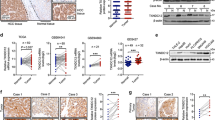
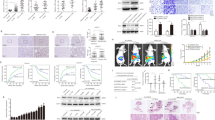
Abstract
Hepatocellular carcinoma (HCC) is one of the most common malignancies and leading causes of cancer-related deaths globally. Despite significant advances in therapy, the molecular mechanisms underlying HCC development and progression remain unclear. Here, we aimed to explore the potential role of PDLIM2 in the development and epithelial-mesenchymal transition (EMT) of HCC via a possible modulation of β-catenin. We first confirmed that PDLIM2 was downregulated in HCC tissues and cells and found lower PDLIM2 expression was associated with worse prognosis in HCC patients. Loss- and gain- of function experiments were performed to evaluate the roles of PDLIM2 and β-catenin in HCC cell proliferation, migration, invasion, EMT, and colony formation. EMT was determined based on the levels of E-cadherin, zonula occludens-1, N-cadherin, and vimentin expression. In vivo, the roles of PDLIM2 and β-catenin in HCC were investigated by using a nude mouse xenograft model. It should be noted that PDLIM2 led to the inhibition of β-catenin activity and its downstream gene expression. Importantly, ectopic PDLIM2 expression inhibited the proliferation, migration, invasion, and EMT of HCC cells by reducing β-catenin expression both in vitro and in vivo, thereby suppressing the occurrence and progression of HCC. Taken together, our results demonstrated that overexpressed PDLIM2 exerts a tumor-suppressive role in HCC by regulating β-catenin. This study suggests that the PDLIM2 may be a promising target for the treatment of HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Caruso S, Nault JC. A dive into the deep heterogeneity of hepatocellular carcinoma. Gastroenterology. 2019;157:1477–9.
Cui H, Zhang Y, Zhang Q, Chen W, Zhao H, Liang J. A comprehensive genome-wide analysis of long noncoding RNA expression profile in hepatocellular carcinoma. Cancer Med 2017;6:2932–41.
He P, Zhang HX, Sun CY, Chen CY, Jiang HQ. Overexpression of SASH1 inhibits the proliferation, invasion, and EMT in hepatocarcinoma cells. Oncol Res. 2016;24:25–32.
Pinto Marques H, Gomes da Silva S, De Martin E, Agopian VG, Martins PN. Emerging biomarkers in HCC patients: Current status. Int J Surg. 2020. https://doi.org/10.1016/j.ijsu.2020.04.043.
Ding GY, Zhu XD, Ji Y, Shi GM, Shen YH, Zhou J, et al. Serum PON1 as a biomarker for the estimation of microvascular invasion in hepatocellular carcinoma. Ann Transl Med. 2020;8:204.
Tanaka T, Grusby MJ, Kaisho T. PDLIM2-mediated termination of transcription factor NF-kappaB activation by intranuclear sequestration and degradation of the p65 subunit. Nat Immunol. 2007;8:584–91.
Sun F, Xiao Y, Qu Z. Oncovirus Kaposi sarcoma herpesvirus (KSHV) represses tumor suppressor PDLIM2 to persistently activate nuclear factor kappaB (NF-kappaB) and STAT3 transcription factors for tumorigenesis and tumor maintenance. J Biol Chem. 2015;290:7362–8.
Oh BY, Cho J, Hong HK, Bae JS, Park WY, Joung JG, et al. Exome and transcriptome sequencing identifies loss of PDLIM2 in metastatic colorectal cancers. Cancer Manag Res. 2017;9:581–9.
Qu Z, Yan P, Fu J, Jiang J, Grusby MJ, Smithgall TE, et al. DNA methylation-dependent repression of PDZ-LIM domain-containing protein 2 in colon cancer and its role as a potential therapeutic target. Cancer Res. 2010;70:1766–72.
Fatima S, Shi X, Lin Z, Chen GQ, Pan XH, Wu JC, et al. 5-Hydroxytryptamine promotes hepatocellular carcinoma proliferation by influencing beta-catenin. Mol Oncol. 2016;10:195–212.
Lai TY, Su CC, Kuo WW, Yeh YL, Kuo WH, Tsai FJ, et al. beta-catenin plays a key role in metastasis of human hepatocellular carcinoma. Oncol Rep. 2011;26:415–22.
Anson M, Crain-Denoyelle AM, Baud V, Chereau F, Gougelet A, Terris B, et al. Oncogenic beta-catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J Clin Invest. 2012;122:586–99.
Sun L, Liu T, Zhang S, Guo K, Liu Y. Oct4 induces EMT through LEF1/beta-catenin dependent WNT signaling pathway in hepatocellular carcinoma. Oncol Lett. 2017;13:2599–606.
Han D, Li J, Wang H, Su X, Hou J, Gu Y, et al. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology 2017;66:1151–64.
Xu B, Cai Z, Zeng Y, Chen L, Du X, Huang A, et al. alpha-Methylacyl-CoA racemase (AMACR) serves as a prognostic biomarker for the early recurrence/metastasis of HCC. J Clin Pathol. 2014;67:974–9.
Wu S, Du R, Gao C, Kang J, Wen J, Sun T. The role of XBP1s in the metastasis and prognosis of hepatocellular carcinoma. Biochem Biophys Res Commun. 2018;500:530–7.
Torrado M, Senatorov VV, Trivedi R, Fariss RN, Tomarev SIPdlim2. a novel PDZ-LIM domain protein, interacts with alpha-actinins and filamin A. Invest Ophthalmol Vis Sci. 2004;45:3955–63.
Cox OT, O’Shea S, Tresse E, Bustamante-Garrido M, Kiran-Deevi R, O’Connor R. IGF-1 receptor and adhesion signaling: an important axis in determining cancer cell phenotype and therapy resistance. Front Endocrinol (Lausanne). 2015;6:106.
Cheng X, Xu X, Chen D, Zhao F, Wang W. Therapeutic potential of targeting the Wnt/beta-catenin signaling pathway in colorectal cancer. Biomed Pharmacother. 2019;110:473–81.
Qu Z, Fu J, Ma H, Zhou J, Jin M, Mapara MY, et al. PDLIM2 restricts Th1 and Th17 differentiation and prevents autoimmune disease. Cell Biosci. 2012;2:23.
de Sousa LM, Dos Santos Alves JM, da Silva Martins C, Pereira KMA, Goes P, Gondim DV. Immunoexpression of canonical Wnt and NF-kappaB signaling pathways in the temporomandibular joint of arthritic rats. Inflamm Res. 2019;68:889–900.
Li C, Wu W, Ding H, Li Q, Xie K. The transcription factor 7 like 2binding protein TIP5 activates betacatenin/transcription factor signaling in hepatocellular carcinoma. Mol Med Rep. 2018;17:7645–51.
Li ZQ, Ding W, Sun SJ, Li J, Pan J, Zhao C, et al. Cyr61/CCN1 is regulated by Wnt/beta-catenin signaling and plays an important role in the progression of hepatocellular carcinoma. PLoS ONE. 2012;7:e35754.
Liu J, Ruan B, You N, Huang Q, Liu W, Dang Z, et al. Downregulation of miR-200a induces EMT phenotypes and CSC-like signatures through targeting the beta-catenin pathway in hepatic oval cells. PLoS ONE. 2013;8:e79409.
Zhang T, Ma Z, Liu L, Sun J, Tang H, Zhang B, et al. DDX39 promotes hepatocellular carcinoma growth and metastasis through activating Wnt/beta-catenin pathway. Cell Death Dis. 2018;9:675.
Acknowledgements
We acknowledge and appreciate our colleagues for their valuable suggestions and technical assistance for this study.
Funding
This study was supported by the National Natural Science Foundation of China (81800557), and China Postdoctoral Science Foundation (2018M631886).
Author information
Authors and Affiliations
Contributions
Xiaoming Jiang and Ying Tang designed the study. Zhe Chu was involved in data collection. Yang Cao and Ying Shi performed the statistical analysis and preparation of figures. Ying Tang and Xu Shi drafted the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Jiang, X., Chu, Z., Cao, Y. et al. PDLIM2 prevents the malignant phenotype of hepatocellular carcinoma cells by negatively regulating β-catenin. Cancer Gene Ther 28, 1113–1124 (2021). https://doi.org/10.1038/s41417-020-00257-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-020-00257-6