Abstract
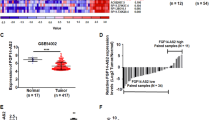
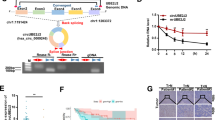
Cervical cancer is a common cause of cancer-related mortality in women. Mounting evidence suggests that long non-coding RNAs (lncRNAs) function vitally in many cancers. In this study, we discovered that the regulation of the heart and neural crest derivatives expressed 2-antisense RNA 1 (HAND2-AS1) in cervical cancer. RT-qPCR was conducted to detect the expression of HAND2-AS1 and microRNA-21-5p (miR-21-5p). The relationship of HAND2-AS1 and miR-21-5p was identified by dual-luciferase reporter gene assay. The roles of HAND2-AS1, miR-21-5p and tissue inhibitor of metalloproteinases-3 (TIMP3) in cervical cancer were accessed via gain- and loss-of-function approaches. The expression of related proteins in the vascular endothelial growth factor A (VEGFA) signaling pathway was detected through Western blot analysis. Finally, xenografts of cervical cancer in nude mice were established to assess the effect of HAND2-AS1 on tumorigenesis in vivo. HAND2-AS1 and TIMP3 were downregulated in cervical cancer, which were identified to be associated with a poor prognosis of patients with cervical cancer. Moreover, HAND2-AS1 was upregulated the expression of TIMP3 through competitively binding to miR-21-5p. Overexpressed HAND2-AS1 or downregulated miR-21-5p inhibited cell proliferation, migration, and invasion while promoting cell apoptosis, in association with increased expression of proteins in VEGFA signaling pathway. These changes were reversed by silencing of TIMP3. Overexpressed HAND2-AS1 reduced the tumor formation ability in nude mice. In summary, HAND2-AS1 may exert inhibitory effects on cervical cancer cell growth and cervical cancer development through its regulation on the miR-21-5p/TIMP3/VEGFA axis. This highlights that HAND2-AS1 may serve as a potential target for cervical cancer diagnosis and treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All the data obtained and/or analyzed during the current study were available from the corresponding authors on reasonable request.
References
Small W Jr, Bacon MA, Bajaj A, Chuang LT, Fisher BJ, Harkenrider MM, et al. Cervical cancer: a global health crisis. Cancer. 2017;123:2404–12.
Barra F, Lorusso D, Leone Roberti Maggiore U, Ditto A, Bogani G, Raspagliesi F, et al. Investigational drugs for the treatment of cervical cancer. Expert Opin Investig Drugs. 2017;26:389–402.
Zhao YB, Wang JH, Chen XX, Wu YZ, Wu Q. Values of three different preoperative regimens in comprehensive treatment for young patients with stage Ib2 cervical cancer. Asian Pac J Cancer Prev. 2012;13:1487–9.
Szczesniak MW, Makalowska I. lncRNA-RNA Interactions across the Human Transcriptome. PLoS ONE. 2016;11:e0150353.
Yang W, Hong L, Xu X, Wang Q, Huang J, Jiang L. LncRNA GAS5 suppresses the tumorigenesis of cervical cancer by downregulating miR-196a and miR-205. Tumour Biol. 2017;39:1010428317711315.
Iden M, Fye S, Li K, Chowdhury T, Ramchandran R, Rader JS. The lncRNA PVT1 contributes to the cervical cancer phenotype and associates with poor patient prognosis. PLoS ONE. 2016;11:e0156274.
Yang X, Wang CC, Lee WYW, Trovik J, Chung TKH, Kwong J. Long non-coding RNA HAND2-AS1 inhibits invasion and metastasis in endometrioid endometrial carcinoma through inactivating neuromedin U. Cancer Lett. 2018;413:23–34.
Lu H, He Y, Lin L, Qi Z, Ma L, Li L, et al. Long non-coding RNA MALAT1 modulates radiosensitivity of HR-HPV+ cervical cancer via sponging miR-145. Tumour Biol. 2016;37:1683–91.
Gao YL, Zhao ZS, Zhang MY, Han LJ, Dong YJ, Xu B. Long noncoding RNA PVT1 facilitates cervical cancer progression via negative regulating of miR-424. Oncol Res. 2017;25:1391–8.
Wu W. MicroRNA: potential targets for the development of novel drugs? Drugs R D. 2010;10:1–8.
Cai L, Wang W, Li X, Dong T, Zhang Q, Zhu B, et al. MicroRNA-21-5p induces the metastatic phenotype of human cervical carcinoma cells in vitro by targeting the von Hippel-Lindau tumor suppressor. Oncol Lett. 2018;15:5213–9.
Zhang Z, Wang J, Wang X, Song W, Shi Y, Zhang L. MicroRNA-21 promotes proliferation, migration, and invasion of cervical cancer through targeting TIMP3. Arch Gynecol Obstet. 2018;297:433–42.
Chen B, Zhang C, Dong P, Guo Y, Mu N. Molecular regulation of cervical cancer growth and invasion by VEGFa. Tumour Biol. 2014;35:11587–93.
Zhu X, Er K, Mao C, Yan Q, Xu H, Zhang Y, et al. miR-203 suppresses tumor growth and angiogenesis by targeting VEGFA in cervical cancer. Cell Physiol Biochem. 2013;32:64–73.
Ginsburg O, Bray F, Coleman MP, Vanderpuye V, Eniu A, Kotha SR, et al. The global burden of women’s cancers: a grand challenge in global health. Lancet. 2017;389:847–60.
Kori M, Yalcin Arga K. Potential biomarkers and therapeutic targets in cervical cancer: Insights from the meta-analysis of transcriptomics data within network biomedicine perspective. PLoS ONE. 2018;13:e0200717.
Peng L, Yuan X, Jiang B, Tang Z, Li GC. LncRNAs: key players and novel insights into cervical cancer. Tumour Biol. 2016;37:2779–88.
Chen S, Xu X, Lu S, Hu B. Long non-coding RNA HAND2-AS1 targets glucose metabolism and inhibits cancer cell proliferation in osteosarcoma. Oncol Lett. 2019;18:1323–9.
Yang Y, Chen L, Gu J, Zhang H, Yuan J, Lian Q, et al. Recurrently deregulated lncRNAs in hepatocellular carcinoma. Nat Commun. 2017;8:14421.
Militello G, Weirick T, John D, Doring C, Dimmeler S, Uchida S. Screening and validation of lncRNAs and circRNAs as miRNA sponges. Brief Bioinform. 2017;18:780–8.
Ergun S, Oztuzcu S. Oncocers: ceRNA-mediated cross-talk by sponging miRNAs in oncogenic pathways. Tumour Biol. 2015;36:3129–36.
Zhang J, Yao T, Wang Y, Yu J, Liu Y, Lin Z. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer Biol Ther. 2016;17:104–13.
Han Y, Xu GX, Lu H, Yu DH, Ren Y, Wang L, et al. Dysregulation of miRNA-21 and their potential as biomarkers for the diagnosis of cervical cancer. Int J Clin Exp Pathol. 2015;8:7131–9.
Song R, Cong L, Ni G, Chen M, Sun H, Sun Y, et al. MicroRNA-195 inhibits the behavior of cervical cancer tumors by directly targeting HDGF. Oncol Lett. 2017;14:767–75.
Lin C, Huang F, Zhang YJ, Tuokan T, Kuerban G. Roles of MiR-101 and its target gene Cox-2 in early diagnosis of cervical cancer in Uygur women. Asian Pac J Cancer Prev. 2014;15:45–8.
Guarino M. Src signaling in cancer invasion. J Cell Physiol. 2010;223:14–26.
Ying L, Hofseth LJ. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res. 2007;67:1407–10.
Liu S, Song L, Yao H, Zhang L, Xu D, Gao F, et al. MiR-375 is epigenetically downregulated by HPV-16 E6 mediated DNMT1 upregulation and modulates EMT of cervical cancer cells by suppressing lncRNA MALAT1. PLoS ONE. 2016;11:e0163460.
Yang JR, Shi MX, Zeng Y. LncRNA HAND2-AS1 inhibits proliferation and promotes apoptosis of chronic myeloid leukemia cells by sponging with micRNA-1275. Eur Rev Med Pharm Sci. 2019;23:2103–11.
Chen S, Wang J. HAND2-AS1 inhibits invasion and metastasis of cervical cancer cells via microRNA-330-5p-mediated LDOC1. Cancer Cell Int. 2019;19:353.
Gong J, Fan H, Deng J, Zhang Q. LncRNA HAND2-AS1 represses cervical cancer progression by interaction with transcription factor E2F4 at the promoter of C16orf74. J Cell Mol Med. 2020;24:6015–27.
Acknowledgements
This study was supported by Youth Fund of Guizhou Provincial People’s Hospital (No. GZSYQN(2019)02). We acknowledge and appreciate our colleagues for their valuable suggestions and technical assistance for this study.
Author information
Authors and Affiliations
Contributions
YG conceived and designed the study. YG, TZ, and WTL collected the data, analyzed the data and wrote the manuscript. YG, ZJZ, and MRQ assisted with the data analyses and participated in the writing of manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
The study protocols were approved by the Ethic Committee of Guizhou Provincial People’s Hospital and performed in line with the Declaration of Helsinki. Animal experiments were conducted in strict accordance with the Guide for Care and Use of Laboratory Animals issued by the National Institutes of Health (USA). The protocol of animal experiments was approved by the Institutional Animal Care and Use Committee of Guizhou Provincial People’s Hospital.
Informed consent
Informed written consent was obtained from each participant prior to the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, Y., Zou, T., Liang, W. et al. Long non-coding RNA HAND2-AS1 delays cervical cancer progression via its regulation on the microRNA-21-5p/TIMP3/VEGFA axis. Cancer Gene Ther 28, 619–633 (2021). https://doi.org/10.1038/s41417-020-00243-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-020-00243-y
This article is cited by
-
HNRNPA2B1-mediated m6A modification of lncRNA MEG3 facilitates tumorigenesis and metastasis of non-small cell lung cancer by regulating miR-21-5p/PTEN axis
Journal of Translational Medicine (2023)
-
A review on the role of HAND2-AS1 in cancer
Clinical and Experimental Medicine (2023)
-
Consequences of aberrated DNA methylation in Colon Adenocarcinoma: a bioinformatic-based multi-approach
BMC Genomic Data (2022)
-
linc00958/miR-185-5p/RSF-1 modulates cisplatin resistance and angiogenesis through AKT1/GSK3β/VEGFA pathway in cervical cancer
Reproductive Biology and Endocrinology (2022)
-
The functions of lncRNAs in the HPV-negative cervical cancer compared with HPV-positive cervical cancer
Apoptosis (2022)