Abstract
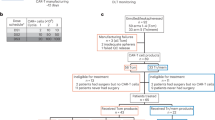
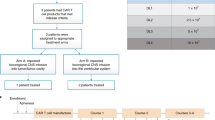
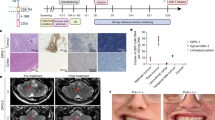
Neural stem cells (NSCs) are tumor tropic and can be genetically modified to produce anti-cancer therapies locally in the brain. In a prior first-in-human study we demonstrated that a single dose of intracerebrally administered allogeneic NSCs, which were retrovirally transduced to express cytosine deaminase (CD), tracked to glioma sites and converted oral 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU). The next step in the clinical development of this NSC-based anti-cancer strategy was to assess the feasibility of administering multiple intracerebral doses of CD-expressing NSCs (CD-NSCs) in patients with recurrent high-grade gliomas. CD-NSCs were given every 2 weeks using an indwelling brain catheter, followed each time by a 7-d course of oral 5-FC (and leucovorin in the final patient cohort). Fifteen evaluable patients received a median of 4 (range 2–10) intracerebral CD-NSC doses; doses were escalated from 50 × 106 to 150 × 106 CD-NSCs. Neuropharmacokinetic data confirmed that CD-NSCs continuously produced 5-FU in the brain during the course of 5-FC. There were no clinical signs of immunogenicity, and only three patients developed anti-NSC antibodies. Our results suggest intracerebral administration of serial doses of CD-NSCs is safe and feasible and identified a recommended dose for phase II testing of 150 × 106 CD-NSCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388:787–96.
Hanus J, Zhao FK, Wang SS. Current therapeutic developments in atrophic age-related macular degeneration. Brit J Ophthalmol. 2016;100:122–7.
Parameswaran S, Krishnakumar S. Pluripotent stem cells: a therapeutic source for age-related macular degeneration. Indian J Ophthalmol. 2017;65:177–83.
Mazzini L, Gelati M, Profico DC, Soraru G, Ferrari D, Copetti M, et al. Results from phase I clinical trial with intraspinal injection of neural stem cells in amyotrophic lateral sclerosis: a long-term outcome. Stem Cells Transl Med. 2019;8:887–97.
Feldman EL, Boulis NM, Hur J, Johe K, Rutkove SB, Federici T, et al. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol. 2014;75:363–73.
Curtis E, Martin JR, Gabel B, Sidhu N, Rzesiewicz TK, Mandeville R, et al. A first-in-human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell. 2018;22:941–50. e6
Pereira IM, Marote A, Salgado AJ, Silva NA. Filling the gap: neural stem cells as a promising therapy for spinal cord injury. Pharmaceuticals. 2019;12:65.
Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–51.
Kendall SE, Najbauer J, Johnston HF, Metz MZ, Li S, Bowers M, et al. Neural stem cell targeting of glioma is dependent on phosphoinositide 3-kinase signaling. Stem Cells. 2008;26:1575–86.
Zhang S, Xie R, Zhao T, Yang X, Han L, Ye F, et al. Neural stem cells preferentially migrate to glioma stem cells and reduce their stemness phenotypes. Int J Oncol. 2014;45:1989–96.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl J Med. 2005;352:987–96.
Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318:2306–16.
Kim SK, Kim SU, Park IH, Bang JH, Aboody KS, Wang KC, et al. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006;12:5550–6.
Aboody KS, Najbauer J, Metz MZ, D’Apuzzo M, Gutova M, Annala AJ, et al. Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci Transl Med. 2013;5:184ra59.
Teng J, Hejazi S, Badr CE, Tannous BA. Systemic anticancer neural stem cells in combination with a cardiac glycoside for glioblastoma therapy. Stem Cells. 2014;32:2021–32.
Seol HJ, Jin J, Seong DH, Joo KM, Kang W, Yang H, et al. Genetically engineered human neural stem cells with rabbit carboxyl esterase can target brain metastasis from breast cancer. Cancer Lett. 2011;311:152–9.
Hong SH, Lee HJ, An J, Lim I, Borlongan C, Aboody KS, et al. Human neural stem cells expressing carboxyl esterase target and inhibit tumor growth of lung cancer brain metastases. Cancer Gene Ther. 2013;20:678–82.
Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–50.
Ahmed AU, Thaci B, Tobias AL, Auffinger B, Zhang L, Cheng Y, et al. A preclinical evaluation of neural stem cell-based cell carrier for targeted antiglioma oncolytic virotherapy. J Natl Cancer Inst. 2013;105:968–77.
Thaci B, Ahmed AU, Ulasov IV, Tobias AL, Han Y, Aboody KS, et al. Pharmacokinetic study of neural stem cell-based cell carrier for oncolytic virotherapy: targeted delivery of the therapeutic payload in an orthotopic brain tumor model. Cancer Gene Ther. 2012;19:431–42.
Portnow J, Synold TW, Badie B, Tirughana R, Lacey SF, D’Apuzzo M, et al. Neural stem cell-based anticancer gene therapy: a first-in-human study in recurrent high-grade glioma patients. Clin Cancer Res. 2017;23:2951–60.
Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology. 2004;24:159–71.
Kim SU. Genetically engineered human neural stem cells for brain repair in neurological diseases. Brain Dev. 2007;29:193–201.
Kim SU, Lee HJ, Park IH, Chu K, Lee ST, Kim M, et al. Human neural stem cells for brain repair. Int J Stem Cells. 2008;1:27–35.
Blakeley J, Portnow J. Microdialysis for assessing intratumoral drug disposition in brain cancers: a tool for rational drug development. Expert Opin Drug Met. 2010;6:1477–91.
Ratain MJ, Mick R, Schilsky RL, Siegler M. Statistical and ethical issues in the design and conduct of phase I and II clinical trials of new anticancer agents. J Natl Cancer Inst. 1993;85:1637–43.
Nadal JC, Van Groeningen CJ, Pinedo HM, Peters GJ. In vivo potentiation of 5-fluorouracil by leucovorin in murine colon carcinoma. Biomed Pharmacother. 1988;42:387–93.
Borner MM, Castiglione M, Bacchi M, Weber W, Herrmann R, Fey MF, et al. The impact of adding low-dose leucovorin to monthly 5-fluorouracil in advanced colorectal carcinoma: results of a phase III trial. Swiss Group for Clinical Cancer Research (SAKK). Ann Oncol. 1998;9:535–41.
Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate by the advanced colorectal cancer meta-analysis project. J Clin Oncol. 1992;10:896–903.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20.
Kim SU, Nagai A, Nakagawa E, Choi HB, Bang JH, Lee HJ, et al. Production and characterization of immortal human neural stem cell line with multipotent differentiation property. Methods Mol Biol. 2008;438:103–21.
Bergenheim AT, Capala J, Roslin M, Henriksson R. Distribution of BPA and metabolic assessment in glioblastoma patients during BNCT treatment: a microdialysis study. J Neurooncol. 2005;71:287–93.
Blakeley JO, Olson J, Grossman SA, He X, Weingart J, Supko JG, et al. Effect of blood brain barrier permeability in recurrent high grade gliomas on the intratumoral pharmacokinetics of methotrexate: a microdialysis study. J Neurooncol. 2009;91:51–8.
Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res. 2009;15:7092–8.
Portnow J, Badie B, Markel S, Liu A, D’Apuzzo M, Frankel P, et al. A neuropharmacokinetic assessment of bafetinib, a second generation dual BCR-Abl/Lyn tyrosine kinase inhibitor, in patients with recurrent high-grade gliomas. Eur J Cancer. 2013;49:1634–40.
Portnow J, Badie B, Liu X, Frankel P, Mi S, Chen M, et al. A pilot microdialysis study in brain tumor patients to assess changes in intracerebral cytokine levels after craniotomy and in response to treatment with a targeted anti-cancer agent. J Neurooncol. 2014;118:169–77.
Brouwer AE, van Kan HJ, Johnson E, Rajanuwong A, Teparrukkul P, Wuthiekanun V, et al. Oral versus intravenous flucytosine in patients with human immunodeficiency virus-associated cryptococcal meningitis. Antimicrob Agents Chemother. 2007;51:1038–42.
Kerr IG, Zimm S, Collins JM, O’Neill D, Poplack DG. Effect of intravenous dose and schedule on cerebrospinal fluid pharmacokinetics of 5-fluorouracil in the monkey. Cancer Res. 1984;44:4929–32.
Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48:2192–202.
Chaichana KL, Kone L, Bettegowda C, Weingart JD, Olivi A, Lim M, et al. Risk of surgical site infection in 401 consecutive patients with glioblastoma with and without carmustine wafer implantation. Neurol Res. 2015;37:717–26.
Uzuka T, Takahashi H, Nakasu Y, Okuda T, Mitsuya K, Hayashi N, et al. Surgical site infection after malignant brain tumor resection: a multicenter study for induction of a basic care bundle. Neurol Med-Chir. 2017;57:542–7.
Qin B, Tanaka R, Ariyama H, Shibata Y, Arita S, Kusaba H, et al. In-vitro differential metabolism and activity of 5-fluorouracil between short-term, high-dose and long-term, low-dose treatments in human squamous carcinoma cells. Anticancer Drugs. 2006;17:439–43.
Calabro-Jones PM, Byfield JE, Ward JF, Sharp TR. Time-dose relationships for 5-fluorouracil cytotoxicity against human epithelial cancer cells in vitro. Cancer Res. 1982;42:4413–20.
Morshed RA, Gutova M, Juliano J, Barish ME, Hawkins-Daarud A, Oganesyan D, et al. Analysis of glioblastoma tumor coverage by oncolytic virus-loaded neural stem cells using MRI-based tracking and histological reconstruction. Cancer Gene Ther. 2015;22:55–61.
Bagci-Onder T, Wakimoto H, Anderegg M, Cameron C, Shah K. A dual PI3K/mTOR inhibitor, PI-103, cooperates with stem cell-delivered TRAIL in experimental glioma models. Cancer Res. 2011;71:154–63.
Hingtgen S, Ren X, Terwilliger E, Classon M, Weissleder R, Shah K. Targeting multiple pathways in gliomas with stem cell and viral delivered S-TRAIL and Temozolomide. Mol Cancer Ther. 2008;7:3575–85.
Frank RT, Edmiston M, Kendall SE, Najbauer J, Cheung CW, Kassa T, et al. Neural stem cells as a novel platform for tumor-specific delivery of therapeutic antibodies. PLoS ONE. 2009;4:e8314.
Mooney R, Roma L, Zhao D, Van Haute D, Garcia E, Kim SU, et al. Neural stem cell-mediated intratumoral delivery of gold nanorods improves photothermal therapy. ACS Nano. 2014;8:12450–60.
Tobias AL, Thaci B, Auffinger B, Rincon E, Balyasnikova IV, Kim CK. et al.The timing of neural stem cell-based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma.Stem Cell Transl Med. 2013;2:655–66.
Acknowledgements
We thank Narine Arabyan, PhD, Keely Walker, PhD, and Alice Tuan, BS, for their editing and/or technical assistance with preparing this manuscript. We also thank Shiny Wu, BS, for her technical support in providing the PK data.
Funding
This research was supported by grants from the United States Food and Drug Administration R01 FD004816 (co-principal investigators: J. Portnow and K.S. Aboody), Phase One Foundation (PI: J. Portnow), The Rosalinde and Arthur Gilbert Foundation, and City of Hope. In addition, research reported in this publication included work performed in the Analytical Pharmacology and Biostatistics and Mathematical Modeling Cores supported by the National Cancer Institute of the National Institutes of Health under grant number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K.S. Aboody is a shareholder and board member of TheraBiologics, Inc.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Portnow, J., Badie, B., Suzette Blanchard, M. et al. Feasibility of intracerebrally administering multiple doses of genetically modified neural stem cells to locally produce chemotherapy in glioma patients. Cancer Gene Ther 28, 294–306 (2021). https://doi.org/10.1038/s41417-020-00219-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-020-00219-y
This article is cited by
-
Mechanistic insights and the clinical prospects of targeted therapies for glioblastoma: a comprehensive review
Experimental Hematology & Oncology (2024)
-
Cryopreservation does not change the performance and characteristics of allogenic mesenchymal stem cells highly over-expressing a cytoplasmic therapeutic transgene for cancer treatment
Stem Cell Research & Therapy (2022)