Abstract
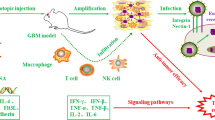
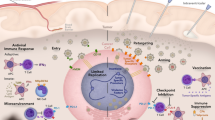
Glioblastoma multiforme is a highly malignant primary brain tumour found in adults and is highlighted as the most devastating among all the other grades of glioma. Well-established standard treatment methods, such as chemotherapy, radiation and surgery, have resulted in modest improvement in the survival of patients. Hence, the arduous search for novel treatments backed by advancements in molecular biology still persists. Glioblastoma has many distinctive characteristics, which makes it a potential candidate for gene therapy. Gene therapy involves the delivery of genetic material of therapeutic use into tumour cells, which produces a specific antitumour response. Moreover, viruses stimulate a vigorous cytotoxic effect, they are easily modifiable and the inherent property of horizontal transfer of genetic material makes them valuable tools for genetic engineering. In this review, we have enlisted the various viral vectors employed in gene therapy for glioblastoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jäkel S, Dimou L. Glial cells and their function in the adult brain: a journey through the history of their ablation. Front Cell Neurosci. 2017;11:1–17.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20.
Wu W, Lamborn KR, Buckner JC, Novotny PJ, Chang SM, O’Fallon JR, et al. Joint NCCTG and NABTC prognostic factors analysis for high-grade recurrent glioma. Neuro Oncol. 2010;12:164–72.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109.
Wen PY, Reardon DA. Neuro-oncology in 2015: Progress in glioma diagnosis, classification and treatment. Nat Rev Neurol. 2016;12:69–70.
Krakstad C, Chekenya M. Survival signalling and apoptosis resistance in glioblastomas: opportunities for targeted therapeutics. Mol Cancer. 2010;9:1–14.
Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15:ii1–56.
Tamimi AF, Juweid M. Epidemiology and outcome of glioblastoma. In: De Vleeschouwer S, editor. Glioblastoma [Internet]. Brisbane (AU): CodonPublications; 2017. Chapter 8. Available from: https://doi.org/10.15586/codon.glioblastoma.2017.ch8. https://www.ncbi.nlm.nih.gov/books/NBK470003.
Bouwens TAM, Trouw LA, Veerhuis R, Dirven CMF, Lamfers MLM, Al-Khawaja H. Complement activation in glioblastoma multiforme pathophysiology: evidence from serum levels and presence of complement activation products in tumor tissue. J Neuroimmunol. 2015;278:271–6.
Young JS, Chmura SJ, Wainwright DA, Yamini B, Peters KB, Lukas RV. Management of glioblastoma in elderly patients. J Neurol Sci. 2017;380:250–5.
Heiland DH, Haaker G, Watzlawick R, Delev D, Masalha W, Franco P, et al. One decade of glioblastoma multiforme surgery in 342 elderly patients: what have we learned? J Neurooncol. 2018;140:385–91.
Schmidinger M, Linzmayer L, Becherer A, Fazeny-Doerner B, Fakhrai N, Prayer D, et al. Psychometric- and quality-of-life assessment in long-term gliblastoma survivors. J Neurooncol. 2003;63:55–61.
Kwiatkowska A, Nandhu M, Behera P, Chiocca E, Viapiano M. Strategies in gene therapy for glioblastoma. Cancers. 2013;5:1271–305.
Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19:764–72.
The Cancer Genome Atlas Research Network, Hayden E, Atlas CG, Institutes USN, Vogelstein B, Cancer I, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061.
Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–53.
Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110:6021–6.
Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–9.
Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009;100:2235–41.
Ozdemir-Kaynak E, Qutub AA, Yesil-Celiktas O. Advances in glioblastoma multiforme treatment: new models for nanoparticle therapy. Front Physiol. 2018;9:170.
Davis ME. GBM treatment overview. Clin J Oncol Nurs. 2016;20:1–14.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Polivka J Jr, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142:164–75.
Kane JR, Miska J, Young JS, Kanojia D, Kim JW, Lesniak MS. Sui generis: gene therapy and delivery systems for the treatment of glioblastoma. Neuro Oncol. 2015;17:ii24–36.
Dixit K, Kumthekar P. Gene delivery in neuro-oncology. Curr Oncol Rep. 2017;19:69.
Murphy AM, Rabkin SD. Current status of gene therapy for brain tumors. Transl Res. 2013;161:339–54.
Cross D, Burmester JK. Gene therapy for cancer treatment: past, present and future. Clin Med Res. 2006;4:218–27.
M Costa P. Viral and non-viral gene therapy for glioblastoma: new insights into the treatment of malignant brain tumors. J Genet Syndr Gene Ther. 2013;4:1–9.
Ram Z, Culver KW, Oshiro EM, Viola JJ, Devroom HL, Otto E, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354.
Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256:1550–2.
Ostertag D, Amundson KK, Espinoza FL, Martin B, Buckley T, Da Silva APG, et al. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro Oncol. 2012;14:145–59.
Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–7.
Bukrinsky MI, Haffar OK. HIV-1 nuclear import: in search of a leader. Front Biosci. 1997;2:d578–87.
Chang CJ, Hsu CC, Yung MC, Chen KY, Tzao C, Wu WF, et al. Enhanced radiosensitivity and radiation-induced apoptosis in glioma CD133-positive cells by knockdown of SirT1 expression. Biochem Biophys Res Commun. 2009;380:236–42.
Cui Q, Yang S, Ye P, Tian E, Sun G, Zhou J, et al. Downregulation of TLX induces TET3 expression and inhibits glioblastoma stem cell self-renewal and tumorigenesis. Nat Commun. 2016;7:10637.
Sánchez-Hernández L, Hernández-Soto J, Vergara P, González RO, Segovia J. Additive effects of the combined expression of soluble forms of GAS1 and PTEN inhibiting glioblastoma growth. Gene Ther. 2018;25:439–49.
Castro MG, Candolfi M, Wilson TJ, Calinescu A, Paran C, Kamran N, et al. Adenoviral vector-mediated gene therapy for gliomas: coming of age. Expert Opin Biol Ther. 2014;14:1241–57.
van Putten EH, Dirven CM, van den Bent MJ, Lamfers ML. Sitimagene ceradenovec: a gene-based drug for the treatment of operable high-grade glioma. Future Oncol. 2010;6:1691–710.
Kurozumi K, Tamiya T, Ono Y, Otsuka S, Kambara H, Adachi Y, et al. Apoptosis induction with 5-fluorocytosine/cytosine deaminase gene therapy for human malignant glioma cells mediated by adenovirus. J Neurooncol. 2004;66:117–27.
Candolfi M, Xiong W, Yagiz K, Liu C, Muhammad AKMG, Puntel M, et al. Gene therapy-mediated delivery of targeted cytotoxins for glioma therapeutics. Proc Natl Acad Sci. 2010;107:20021–6.
Salem A, Farrokhi C, Lowenstein PR, Puntel M, Curtin JF, Bondale NS, et al. A novel bicistronic high-capacity gutless adenovirus vector that drives constitutive expression of herpes simplex virus type 1 thymidine kinase and tet-inducible expression of Flt3L for glioma therapeutics. J Virol. 2010;84:6007–17.
Ishida J, Kumon H, Oka T, Kurozumi K, Shimazu Y, Watanabe M, et al. Integrin antagonist augments the therapeutic effect of adenovirus-mediated REIC/Dkk-3 gene therapy for malignant glioma. Gene Ther. 2014;22:146–54.
Lakka SS, Rajan M, Gondi C, Yanamandra N, Chandrasekar N, Jasti SL, et al. Adenovirus-mediated expression of antisense MMP-9 in glioma cells inhibits tumor growth and invasion. Oncogene. 2002;21:8011.
Kaliberov SA, Kaliberova LN, Yan H, Kapoor V, Hallahan DE. Retargeted adenoviruses for radiation-guided gene delivery. Cancer Gene Ther. 2016;23:303–14.
Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–6.
Hoggan MD, Blacklow NR, Rowe WP. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, andimmunological characteristics. Proc Natl Acad Sci USA. 1966;55:1467.
Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72:309–19.
Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–8.
Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–27.
GuhaSarkar D, Neiswender J, Su Q, Gao G, Sena-Esteves M. Intracranial AAV-IFN-β gene therapy eliminates invasive xenograft glioblastoma and improves survival in orthotopic syngeneic murine model. Mol Oncol. 2017;11:180–93.
Zhang C, Yao T, Zheng Y, Li Z, Zhang Q, Zhang L, et al. Development of next generation adeno-associated viral vectors capable of selective tropism and efficient gene delivery. Biomaterials. 2016;80:134–45.
Ma HI, Hueng DY, Shui HA, Han JM, Wang CH, Lai YH, et al. Intratumoral decorin gene delivery by AAV vector inhibits brain glioblastomas and prolongs survival of animals by inducing cell differentiation. Int J Mol Sci. 2014;15:4393–414.
Ständer M, Naumann U, Dumitrescu L, Heneka M, Löschmann P, Gulbins E, et al. Decorin gene transfer-mediated suppression of TGF-β synthesis abrogates experimental malignant glioma growth in vivo. Gene Ther. 1998;5:1187–94.
Meijer DH, Maguire CA, Leroy SG, Sena-Esteves M. Controlling brain tumor growth by intraventricular administration of an AAV vector encoding IFN-Β. Cancer Gene Ther. 2009;16:664–71.
Burges HD, Croizier G, Huber J. A review of safety tests on baculoviruses. Entomophaga. 1980;25:329–39.
Wang CY, Li F, Yang Y, Guo HY, Wu CX, Wang S. Recombinant baculovirus containing the diphtheria toxin A gene for malignant glioma therapy. Cancer Res. 2006;66:5798–806.
Wu C, Lin J, Hong M, Choudhury Y, Balani P, Leung D, et al. Combinatorial control of suicide gene expression by tissue-specific promoter and microrna regulation for cancer therapy. Mol Ther. 2009;17:2058–66.
Balani P, Boulaire J, Zhao Y, Zeng J, Lin J, Wang S. High mobility group box2 promoter-controlled suicide gene expression enables targeted glioblastoma treatment. Mol Ther. 2009;17:1003–11.
Guo H, Choudhury Y, Yang J, Chen C, Tay FC, Lim TM, et al. Antiglioma effects of combined use of a baculovirual vector expressing wild-type p53 and sodium butyrate. J Gene Med. 2011;13:26–36.
Chao CN, Yang YH, Wu MS, Chou MC, Fang CY, Lin MC, et al. Gene therapy for human glioblastoma using neurotropic JC virus-like particles as a gene delivery vector. Sci Rep. 2018;8:1–11.
Foreman PM, Friedman GK, Cassady KA, Markert JM. Oncolytic virotherapy for the treatment of malignant glioma. Neurotherapeutics. 2017;14:333–44.
Liu TC, Kirn D. Gene therapy progress and prospects cancer: oncolytic viruses. Gene Ther. 2008;15:877–84.
Dautzenberg IJC, Van Den Hengel SK, De Vrij J, Ravesloot L, Cramer SJ, Hong SS, et al. Baculovirus-assisted reovirus infection in monolayer and spheroid cultures of glioma cells. Sci Rep. 2017;7:17654.
Rommelaere J, Geletneky K, Angelova AL, Daeffler L, Dinsart C, Kiprianova I, et al. Oncolytic parvoviruses as cancer therapeutics. Cytokine Growth Factor Rev. 2010;21:185–95.
Geletneky K, Kiprianova I, Ayache A, Koch R, Herrero Y Calle M, et al. Regression of advanced rat and human gliomas by local or systemic treatment with oncolytic parvovirus H-1 in rat models. Neuro Oncol. 2010;12:804–14.
Parker JN, Bauer DF, Cody JJ, Markert JM. Oncolytic viral therapy of malignant glioma. Neurotherapeutics. 2009;6:558–69.
Phuong LK, Allen C, Peng KW, Giannini C, Greiner S, TenEyck CJ, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–9.
Allen C, Paraskevakou G, Liu C, Iankov ID, Msaouel P, Zollman P, et al. Oncolytic measles virus strains in the treatment of gliomas. Expert Opin Biol Ther. 2008;8:213–20.
McKenzie BA, Zemp FJ, Pisklakova A, Narendran A, McFadden G, Lun X, et al. In vitro screen of a small molecule inhibitor drug library identifies multiple compounds that synergize with oncolytic myxoma virus against human brain tumor-initiating cells. Neuro Oncol. 2015;17:1086–94.
Li K, Hu C, Xing F, Gao M, Liang J, Xiao X, et al. Deficiency of the IRE1α-autophagy axis enhances the antitumor effects of the oncolytic virus M1. J Virol. 2017;92:e01331–17. pii
Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7:33–40.
Zhang W, Cao S, Martin JL, Mueller JD, Mansky LM. Morphology and ultrastructure of retrovirus particles. AIMS Biophys. 2015;2:343.
Caffery B, Lee J, Alexander-Bryant A. Vectors for glioblastoma gene therapy: viral & non-viral delivery strategies. Nanomaterials. 2019;9:105.
Denard J, Rundwasser S, Laroudie N, Gonnet F, Naldini L, Radrizzani M, et al. Quantitative proteomic analysis of lentiviral vectors using 2-DE. Proteomics. 2009;9:3666–76.
Tolmachov O, Tolmachova T, Al-Allaf FA. Designing lentiviral gene vectors. InViral gene therapy. London, United Kingdom: Intech Open Limited; 2012.
Rux JJ, Burnett RM. Adenovirus structure. Hum Gene Ther. 2004;15:1167–76.
Stephen SL, Montini E, Sivanandam VG, Al-Dhalimy M, Kestler HA, Finegold M, et al. Chromosomal integration of adenoviral vector DNA in vivo. J Virol. 2010;84:9987–94.
Wirth T, Samaranayake H, Pikkarainen J, Määttä AM, Ylä-Herttuala S. Clinical trials for glioblastoma multiforme using adenoviral vectors. Curr Opin Mol Ther. 2009;11:485–92.
Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21:583–93.
Gao Y, Ng SSM, Chau DHW, Yao H, Yang C, Man K, et al. Development of recombinant adeno-associated virus and adenovirus cocktail system for efficient hTERTC27 polypeptide-mediated cancer gene therapy. Cancer Gene Ther. 2008;15:723–32.
Merrihew RV, Clay WC, Condreay JP, Witherspoon SM, Dallas WS, Kost TA. Chromosomal integration of transduced recombinant baculovirus DNA in mammalian cells. J Virol. 2002;75:903–9.
Funding
This work is supported by an extramural funding from the Department of Biotechnology, Government of India (Sanction no: BT/PR19625/MED/30/1703/2016) awarded to DS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Manikandan, C., Kaushik, A. & Sen, D. Viral vector: potential therapeutic for glioblastoma multiforme. Cancer Gene Ther 27, 270–279 (2020). https://doi.org/10.1038/s41417-019-0124-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-019-0124-8
This article is cited by
-
Overcoming the blood–brain barrier for the therapy of malignant brain tumor: current status and prospects of drug delivery approaches
Journal of Nanobiotechnology (2022)
-
Effects of oncolytic viruses and viral vectors on immunity in glioblastoma
Gene Therapy (2022)
-
UPF1/circRPPH1/ATF3 feedback loop promotes the malignant phenotype and stemness of GSCs
Cell Death & Disease (2022)