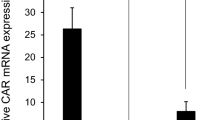
Abstract
Intravesical BCG is a highly effective treatment for high-grade nonmuscle invasive bladder cancer and carcinoma in situ (CIS); however, for patients who are either resistant or become unresponsive to BCG therapy there is a need for alternative treatment approaches. This study examined the safety and feasibility of intravesically administered recombinant fowlpox virus encoding GM-CSF (Arm A) or TRICOM (Arm B); and the local and systemic immunologic responses generated to the vector(s). Twenty bladder cancer patients scheduled for cystectomy as their standard of care received preoperatively four weekly doses of intravesical recombinant fowlpox. Treatment was well tolerated, however, three patients experienced transient elevations of liver transaminases, with one rising to the level of a DLT. Cystectomy derived tumor and normal bladder mucosa demonstrated mRNA for the virally encoded LacZ gene supporting effective infection/transfection. Detected serum antibody to the LacZ encoding β-galactosidase indicated successful expression of vector-encoding gene products and the ability to immunize via the bladder site. H&E and IHC using a panel of immune cell specific antigens demonstrated immune cell infiltration of the bladder wall. These findings demonstrate good safety profile, successful infection/transfection, ability to generate systemic immune response, and local recruitment of immune cell populations with intravesical administration of fowlpox-based constructs encoding for GM-CSF(rF-GM-CSF) or TRICOM (rF-TRICOM), and support further evaluation of this treatment modality for bladder cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
Alfred Witjes J, Lebret T, Comperat EM, Cowan NC, De Santis M, Bruins HM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71:462–75.
Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639–53.
Freyne B, Marchant A, Curtis N. BCG-associated heterologous immunity, a historical perspective: experimental models and immunological mechanisms. Trans R Soc Trop Med Hyg. 2015;109:46–51.
Cookson MS, Herr HW, Zhang ZF, Soloway S, Sogani PC, Fair WR. et al. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol. 1997;158:62–7.
Ahn JJ, Ghandour RA, McKiernan JM. New agents for bacillus Calmette-Guerin-refractory nonmuscle invasive bladder cancer. Curr Opin Urol. 2014;24:540–5.
Herr HW, Milan TN, Dalbagni G. BCG-refractory vs. BCG-relapsing non-muscle-invasive bladder cancer: a prospective cohort outcomes study. Urol Oncol. 2015;33:108 e1–4.
Tyson MD 2nd, Barocas DA. Quality of Life After Radical Cystectomy. Urol Clin N Am. 2018;45:249–56.
Lee SS, Eisenlohr LC, McCue PA, Mastrangelo MJ, Lattime EC. Intravesical gene therapy: in vivo gene transfer using recombinant vaccinia virus vectors. Cancer Res. 1994;54:3325–8.
Mastrangelo MJ, Eisenlohr LC, Gomella L, Lattime EC. Poxvirus vectors: orphaned and underappreciated. J Clin Investig. 2000;105:1031–4.
Gomella LG, Mastrangelo MJ, McCue PA, Maguire HJ, Mulholland SG, Lattime EC. et al. Phase I study of intravesical vaccinia virus as a vector for gene therapy of bladder cancer. J Urol. 2001;166:1291–5.
Schlom J, Hodge JW. The diversity of T-cell co-stimulation in the induction of antitumor immunity. Immunol Rev. 1999;170:73–84.
Grosenbach DW, Barrientos JC, Schlom J, Hodge JW. Synergy of vaccine strategies to amplify antigen-specific immune responses and antitumor effects. Cancer Res. 2001;61:4497–505.
Hodge JW, et al. Admixture of a recombinant vaccinia virus containing the gene for the costimulatory molecule B7 and a recombinant vaccinia virus containing a tumor-associated antigen gene results in enhanced specific T-cell responses and antitumor immunity. Cancer Res. 1995;55:3598–603.
Kass E, Panicali DL, Mazzara G, Schlom J, Greiner JW. Granulocyte/macrophage-colony stimulating factor produced by recombinant avian poxviruses enriches the regional lymph nodes with antigen-presenting cells and acts as an immunoadjuvant. Cancer Res. 2001;61:206–14.
Mastrangelo MJ, Maguire HC Jr., Eisenlohr LC, Laughlin CE, Monken CE, McCue PA, et al. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999;6:409–22.
Deguchi M, Aiba S, Ohtani H, Nagura H, Tagami H. Comparison of the distribution and numbers of antigen-presenting cells among T-lymphocyte-mediated dermatoses: CD1a+, factor XIIIa+, and CD68+ cells in eczematous dermatitis, psoriasis, lichen planus and graft-versus-host disease. Arch Dermatol Res. 2002;294:297–302.
Anastasiadis A, de Reijke TM. Best practice in the treatment of nonmuscle invasive bladder cancer. Ther Adv Urol. 2012;4:13–32.
Mastrangelo MJ, Maguire HC Jr., McCue PA, Lee SS, Alexander A, Nazarian LN, et al. A pilot study demonstrating the feasibility of using intratumoral vaccinia injections as a vector for gene transfer. Vaccine Res. 1995;4:55–69.
Conry RM, Westbrook B, McKee S, Norwood TG. Talimogene laherparepvec: first in class oncolytic virotherapy. Hum Vaccin Immunother. 2018;14:839–46.
Noyes K, Singer EA, Messing EM. Healthcare economics of bladder cancer: cost-enhancing and cost-reducing factors. Curr Opin Urol. 2008;18:533–9.
Di Lorenzo G, Perdona S, Damiano R, Faiella A, Cantiello F, Pignata S, et al. Gemcitabine versus bacille Calmette-Guerin after initial bacille Calmette-Guerin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer. 2010;116:1893–900.
Skinner EC, Goldman B, Sakr WA, Petrylak DP, Lenz HJ, Lee CT, et al. SWOG S0353: Phase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical bacillus Calmette-Guerin. J Urol. 2013;190:1200–4.
Addeo R, Caraglia M, Bellini S, Abbruzzese A, Vincenzi B, Montella L, et al. Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. J Clin Oncol. 2010;28:543–8.
Messing EM, Tangen CM, Lerner SP, Sahasrabudhe DM, Koppie TM, Wood DP Jr., et al. Effect of intravesical instillation of gemcitabine vs saline immediately following resection of suspected low-grade non-muscle-invasive bladder cancer on tumor recurrence: SWOG S0337 randomized clinical trial. JAMA. 2018;319:1880–8.
Joudi FN, Smith BJ, O’Donnell MA. National BCGIPIG. Final results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alpha-2B for reducing recurrence of superficial bladder cancer. Urol Oncol. 2006;24:344–8.
Shore ND, Boorjian SA, Canter DJ, Ogan K, Karsh LI, Downs TM, et al. Intravesical rAd-IFNalpha/Syn3 for patients with high-grade, bacillus Calmette-Guerin-refractory or relapsed non-muscle-invasive bladder cancer: A Phase II randomized study. J Clin Oncol. 2017;35:3410–6.
Dinney CP, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guerin. Urol Oncol. 2013;31:1635–42.
Steinberg G, Bahnson R, Brosman S, Middleton R, Wajsman Z, Wehle M. Efficacy and safety of valrubicin for the treatment of bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The valrubicin study group. J Urol. 2000;163:761–7.
Barlow LJ, McKiernan JM, Benson MC. Long-term survival outcomes with intravesical docetaxel for recurrent nonmuscle invasive bladder cancer after previous bacillus Calmette-Guerin therapy. J Urol. 2013;189:834–9.
Acknowledgements
Studies made use of the Rutgers Cancer Institute of New Jersey Biospecimen and Immunohistochemistry and Research Pharmacy, Shared Resources.
Funding
This trial was supported by the Cancer Therapy Evaluation Program (CTEP), a division of the National Cancer Institute (NCI/CTEP #5585, U01CA132194), R21CA121589, and a grant from the National Cancer Institute (P30CA072720). The fowlpox vaccine, rF-GM-CSF (NSC 707299) and rF-TRICOM (NSC 710658), was manufactured by Therion Biologics Corporation and supplied by the Pharmaceutical Management Branch, CTEP, DCTD, NCI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ECL is an inventor of the patented recombinant vaccinia GM-CSF that has been licensed to Sillajen and is being studied as JX-594 (Pexa-Vec). As such, he derives royalties and licensing fees from the Thomas Jefferson University where the patent is held. EAS receives research funding from Astellas/Medivation. The remaining authors declared no potential conflicts.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Portal, D.E., Weiss, R.E., Wojtowicz, M. et al. Phase I neoadjuvant study of intravesical recombinant fowlpox-GM-CSF (rF-GM-CSF) or fowlpox-TRICOM (rF-TRICOM) in patients with bladder carcinoma. Cancer Gene Ther 27, 438–447 (2020). https://doi.org/10.1038/s41417-019-0112-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-019-0112-z