Abstract
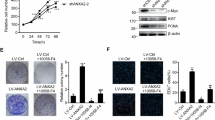
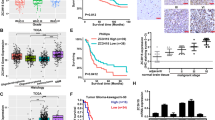
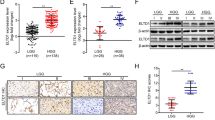
Many efforts have been taken to develop molecule target for cancer therapy. 14-3-3zeta protein has emerged as a critical regulator of diverse cellular pathways in multiple cancers. Furthermore, 14-3-3zeta expression was elevated and a predictor of poor prognosis in glioblastoma. However, there is no information to evaluate the potential effects of 14-3-3zeta RNAi in glioblastoma. The relationship between 14-3-3zeta expression and cell proliferation and apoptosis was tested in primary glioblastoma samples. Through an RNAi approach using human glioblastoma cells as a model system, we demonstrated the role of 14-3-3zeta in glioblastoma proliferation, apoptosis, invasion and tumor growth. The expression of 14-3-3zeta in glioblastoma stem cells was also investigated by immunostaining. The apoptosis was significantly higher in 14-3-3zeta-negative group than in positive group. 14-3-3zeta immunoreactivity score was negatively correlated with the apoptosis, and positively with proliferation in human specimens. 14-3-3zeta RNAi reduced cell proliferation, induced apoptosis, decreased the invasive capability and colony-formation, and impaired the growth of glioblastoma xenografts in nude mice. Moreover, 14-3-3zeta was positively expressed in glioblastoma stem cells. Our data highlight the importance of 14-3-3zeta in glioblastoma and identify 14-3-3zeta as a potential molecular target for glioblastoma treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Perry JR, Laperriere N, O’Collaghan CJ, Brandes AA, Menten J, Phillips C, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376:1027–37.
Park JK, Hodges T, Arko L, Shen M, Dello lacono D, McNabb A, et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol. 2010;28:3838–43.
Felsberg J, Rapp M, Loeser S, Fimmers R, Stummer W, Goeppert M, et al. Prognostic significance of molecular markers and extent of resection in primary glioblastoma patients. Clin Cancer Res. 2009;15:6683–93.
Ferl RJ, Manak MS, Reyes MF. The 14-3-3s. Genome Biol. 2002;3:S3010.
Martin H, Patel Y, Jones D, Howell S, Robinson K, Aitken A. Antibodies against the major brain isoforms of 14-3-3 protein: an antibody specific for the N-acetylated amino-terminus of a protein. FEBS Lett. 1993;336:189.
Muslin AJ, Tanner JW, Allen PM, Shaw AS. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996;84:889–97.
Nishimura Y, Komatsu S, Ichikawa D, Nagata H, Hirajima S, Takeshita H, et al. Overexpression of YWHAZ relates to tumor cell proliferation and malignant outcome of gastric carcinoma. Br J Cancer. 2013;108:1324–31.
Zhao GY, Ding JY, Lu CL, Lin ZW, Guo J. The overexpression of 14-3-3zeta and Hsp27 promotes non-small cell lung cancer progression. Cancer. 2014;120:652–63.
Kittirat Y, Techasen A, Thongchot S, Loilome W, Thanan R, Yongvanit P, et al. Suppression of 14-3-3zeta in cholangiocarcinoma cells inhibits proliferation through attenuated Akt activity, enhancing chemosensitivity to gemcitabine. Oncol Lett. 2018;15:347–53.
Tang Y, Wang R, Zhang Y, Lin S, Qiao N, Sun Z, et al. Co-upregulation of 14-3-3zeta and P-Akt is associated with oncogenesis and recurrence of hepatocellular carcinoma. Cell Physiol Biochem. 2018;10:1097–107.
Cao W, Yang X, Zhou J, Teng Z, Cao L, Zhang X, et al. Targeting 14-3-3 protein, difopein induces apoptosis of human glioma cells and suppresses tumor growth in mice. Apoptosis. 2010;15:230–41.
Yang X, Cao W, Lin H, Zhang W, Lin W, Cao L, et al. Isoform-specific expression of 14-3-3 proteins in human astrocytoma. J Neurol Sci. 2009;276:54–9.
Cao L, Cao W, Zhang W, Lin H, Yang X, Zhen H, et al. Identification of 14-3-3 protein isoforms in human astrocytoma by immunohistochemistry. Neurosci Lett. 2008;432:94–9.
Yang X, Zhou J, Cao W, Zhang W, Zhang X, Lin W, et al. 14-3-3zeta positive expression is associated with a poor prognosis in patients with glioblastoma. Neurosurgery. 2011;68:932–8.
Yang X, Cao W, Zhang L, Zhang W, Zhang X, Lin H. Targeting 14-3-3zeta in cancer therapy. Cancer Gene Ther. 2012;19:153–9.
Neal CL, Yao J, Yang W, Zhou X, Nguyen NT, Lu J, et al. 14-3-3zeta overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res. 2009;69:3425–32.
Bajpai U, Sharma R, Kausar T, Dattagupta S, Chattopadhayay TK, Ralhan R. Clinical significance of 14-3-3zeta in human esophageal cancer. Int J Biol Markers. 2008;23:231–7.
Lin M, Morrison CD, Jones S, Mohamed N, Bacher J, Plass C. Copy number gain and oncogenic activity of YWHAZ/14-3-3zeta in head and neck squamous cell carcinoma. Int J Cancer. 2009;125:603–11.
Matta A, Bahadur S, Duggal R, Gupta SD, Ralhan R. Over-expression of 14-3-3zeta is an early event in oral cancer. BMC Cancer. 2007;7:169.
Matta A, DeSouza LV, Ralhan R, Siu KW. Small interfering RNA targeting 14-3-3zeta increases efficacy of chemotherapeutic agents in head and neck cancer cells. Mol Cancer Ther. 2010;9:2676–88.
Li Z, Zhao J, Du Y, Park HR, Sun SY, Bernal-Mizrachi L, et al. Down-regulation of 14-3-3zeta suppresses anchorage-independent growth of lung cancer cells through anoikis activation. Proc Natl Acad Sci USA. 2008;105:162–7.
Lu J, Guo H, Treekitkarnmongkol W, Li P, Zhang J, Shi B, et al. 14-3-3zeta Cooperates with ErbB2 to promote ductal carcinoma in situ progression to invasive breast cancer by inducing epithelial–mesenchymal transition. Cancer Cell. 2009;16:195–207.
Fan T, Li R, Todd NW, Qiu Q, Fang HB, Wang H, et al. Up-regulation of 14-3-3zeta in lung cancer and its implication as prognostic and therapeutic target. Cancer Res. 2007;67:7901–6.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20.
Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21.
Mao XG, Zhang X, Xue XY, Guo G, Wang P, Zhang W, et al. Brain tumor stem-like cells identified by neural stem cell marker CD15. Transl Oncol. 2009;2:247–57.
Lin W, Zhang J, Zhang J, Liu X, Fei Z, Li X, et al. RNAi-mediated inhibition of MSP58 decreases tumour growth, migration and invasion in a human glioma cell line. J Cell Mol Med. 2009;13:4608–22.
Hong HY, Jeon WK, Bae EJ, Kim ST, Lee HJ, Kim SJ, et al. 14-3-3 sigma and 14-3-3 zeta plays an opposite role in cell growth inhibition mediated by transforming growth factor-beta 1. Mol Cells. 2010;29:305–9.
Li Y, Zou L, Li Q, Haibe-Kains B, Tian R, Li Y, et al. Amplification of LAPTM4B and YWHAZ contributes to chemotherapy resistance and recurrence of breast cancer. Nat Med. 2010;16:214–8.
Neal CL, Xu J, Li P, Mori S, Yang J, Neal NN, et al. Overexpression of 14-3-3zeta in cancer cells activates PI3K via binding the p85 regulatory subunit. Oncogene. 2012;31:897–906.
Mellai M, Caldera V, Patrucco A, Annovazzi L, Schiffer D. Survivin expression in glioblastomas correlates with proliferation, but not with apoptosis. Anticancer Res. 2008;28:109–18.
Momota H, Shih AH, Edgar MA, Holland EC. c-Myc and beta-catenin cooperate with loss of p53 to generate multiple members of the primitive neuroectodermal tumor family in mice. Oncogene. 2008;27:4392–401.
Pu P, Zhang Z, Kang C, Jiang R, Jia Z, Wang G, et al. Downregulation of Wnt2 and beta-catenin by siRNA suppresses malignant glioma cell growth. Cancer Gene Ther. 2009;16:351–61.
Schonthal AH. Exploiting cyclooxygenase-(in)dependent properties of COX-2 inhibitors for malignant glioma therapy. Anticancer Agents Med Chem. 2010;10:450–61.
Shirai K, Suzuki Y, Oka K, Noda SE, Katoh H, Suzuki Y, et al. Nuclear surviving expression predicts poorer prognosis in glioblastoma. J Neurooncol. 2009;91:353–8.
Zhang LY, Jiang LN, Li FF, Li H, Liu F, Gu Y, et al. Reduced beta-catenin expression is associated with good prognosis in Astrocytoma. Pathol Oncol Res. 2010;16:253–7.
Shono T, Tofilon PJ, Bruner JM, Owolabi O, Lang FF. Cyclooxygenase-2 expression in human gliomas: prognostic significance and molecular correlations. Cancer Res. 2001;61:4375–81.
Mils V, Baldin V, Goubin F, Pinta I, Papin C, Waye M, et al. Specific interaction between 14-3-3 isoforms and the human CDC25B phosphatase. Oncogene. 2000;19:1257–65.
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkin C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8.
Tian Q, He XC, Hood L, Li L. Bridging the BMP and Wnt pathways by PI3 kinase/Akt and 14-3-3zeta. Cell Cycle. 2005;4:215–6.
Tian Q, Feetham MC, Tao WA, He XC, Li L, Aebersold R, et al. Proteomic analysis identifies that 14-3-3zeta interacts with beta-catenin and facilitates its activation by Akt. Proc Natl Acad Sci USA. 2004;101:15370–5.
Lee YK, Hur W, Lee SW, Hong SW, Kim SW, Choi JE, et al. Knockdown of 14-3-3zeta enhances radiosensitivity and radio-induced apoptosis in CD133(+) liver cancer stem cell. Exp Mol Med. 2014;46:e77.
Acknowledgements
The authors gratefully acknowledge Mauting Lin (Cancer Research Institute, Helen Diller Family Comprehensive Cancer Center, University of California San Francisco) for his helpful communication and Bo Wang’s (Department of Epidemiology, School of Military Preventive Medicine in our university) help in statistical analysis. The authors thank Jingrong Hu (Department of Immune in our university) for assistance in flow cytometry and Wanjuan Yang for the preparation of material for this research.
Funding
Supported by the National Natural Science Foundation of China (Nos. 39970752, 81472357, and 81072083) and Scientific and Technological Project of ShaanXi Province (No. 2008K09-09).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yang, X., Cao, W., Wang, X. et al. Down-regulation of 14-3-3zeta reduces proliferation and increases apoptosis in human glioblastoma. Cancer Gene Ther 27, 399–411 (2020). https://doi.org/10.1038/s41417-019-0097-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-019-0097-7
This article is cited by
-
Proteomic analysis of extracellular vesicles from tick hemolymph and uptake of extracellular vesicles by salivary glands and ovary cells
Parasites & Vectors (2023)
-
KHDRBS3 accelerates glycolysis and promotes malignancy of hepatocellular carcinoma via upregulating 14-3-3ζ
Cancer Cell International (2023)