Abstract
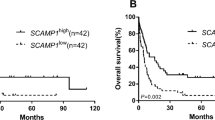
Acute myeloid leukemia (AML) is a hematological malignancy characterized by the proliferation of immature myeloid cells, with impaired differentiation and maturation. Pyruvate dehydrogenase kinase (PDK) is a pyruvate dehydrogenase complex (PDC) phosphatase inhibitor that enhances cell glycolysis and facilitates tumor cell proliferation. Inhibition of its activity can induce apoptosis of tumor cells. Currently, little is known about the role of PDKs in AML. Therefore, we screened The Cancer Genome Atlas (TCGA) database for de novo AML patients with complete clinical information and PDK family expression data, and 84 patients were included for the study. These patients did not undergo allogeneic hematopoietic stem cell transplantation (allo-HSCT). Univariate analysis showed that high expression of PDK2 was associated with shorter EFS (P = 0.047), and high expression of PDK3 was associated with shorter OS (P = 0.026). In multivariate analysis, high expression of PDK3 was an independent risk factor for EFS and OS (P < 0.05). In another TCGA cohort of AML patients who underwent allo-HSCT (n = 71), PDK expression was not associated with OS (all P > 0.05). Our results indicated that high expressions of PDK2 and PDK3, especially the latter, were poor prognostic factors of AML, and the effect could be overcome by allo-HSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441.
Tallman MS, Gilliland DG, R JM. Drug therapy for acutemyeloid leukemia. Blood. 2005;106:1154–63.
Ohtake S, Miyawaki S, Fujita H, Kiyoi H, Shinagawa K, Usui N, et al. Randomized study of induction therapy comparing standard-dose idarubicin with high-dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: the JALSG AML201 Study. Blood. 2011;117:2358–65.
Wu S, Dai Y, Zhang Y, Wang X, Wang L, Ma D, et al. Mutational spectrum and prognostic stratification of intermediate-risk acute myeloid leukemia. Cancer Gene Ther. 2018;25:207–13.
Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100:4325–36.
Schnittger S1, Schoch C, Kern W, Mecucci C, Tschulik C, Martelli MF, et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood. 2005;106:3733–9.
Preudhomme C, Sagot C, Boissel N, Cayuela JM, Tigaud I, de Botton S, et al. Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: a study from the Acute Leukemia French Association (ALFA). Blood. 2002;100:2717–23.
Pabst T1, Eyholzer M, Fos J, M BU. Heterogeneity within AML with CEBPA mutations; only CEBPA double mutations, but not single CEBPA mutations are associated with favourable prognosis. Br J Cancer. 2009;100:1343–6.
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–9.
Döhner K, Schlenk RF, Habdank M, Scholl C, Rücker FG, Corbacioglu A, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005;106:3740–6.
Ryotokuji T, Yamaguchi H, Ueki T, Usuki K, Kurosawa S, Kobayashi Y, et al. Clinical characteristics and prognosis of acute myeloid leukemia associated with DNA-methylation regulatory gene mutations. Haematologica. 2016;101:1074–81.
Terada K, Yamaguchi H, Ueki T, Usuki K, Kobayashi Y, Tajika K, et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosomes Cancer. 2018;57:401–8.
Gudi R, Bowker-Kinley MM, Kedishvili NY, Zhao Y, P KM. Diversity of the pyruvate dehydrogenase kinase gene family in humans. J Biol Chem. 1995;270:28989–94.
Korotchkina LG, P MS. Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase. J Biol Chem. 2001;276:37223–9.
Pouysségur J, Dayan F, M NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–43.
Bowker-Kinley MM, Davis WI, Wu P, Harris RA, P KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329(Pt 1):191–6.
Pearn L, Fisher J, Burnett AK, D RL. The role of PKC and PDK1 in monocyte lineage specification by Ras. Blood. 2007;109:4461–9.
Yoon JS, Won YW, Kim SJ, Oh SJ, Kim ES, Kim BK, et al. Anti-leukemic effect of 2-pyrone derivatives via MAPK and PI3 kinase pathways. Invest New Drugs. 2012;30:2284–93.
Wigfield SM, Winter SC, Giatromanolaki A, Taylor J, Koukourakis ML, H AL. PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer. Br J Cancer. 2008;98:1975–84.
Zabkiewicz J, Pearn L, Hills RK, Morgan RG, Tonks A, Burnett AK, et al. The PDK1 master kinase is over-expressed in acute myeloid leukemia and promotes PKC-mediated survival of leukemic blasts. Haematologica. 2014;99:858–64.
Yang J, Cao Q, Zhang H, Hao L, Zhou D, Gan Z, et al. Targeted reversal and phosphorescence lifetime imaging of cancer cell metabolism via a theranostic rhenium(I)-DCA conjugate. Biomaterials. 2018;176:94–105.
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–3.
Zhang SL, Hu X, Zhang W, Yao H1, T KY. Development of pyruvate dehydrogenase kinase inhibitors in medicinal chemistry with particular emphasis as anticancer agents. Drug Discov Today. 2015;20:1112–9.
Kaplon J, Zheng L, Meissl K, Chaneton B, Selivanov VA, Mackay G, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 2013;498:109–12.
Pate KT, Stringari C, Sprowl-Tanio S, Wang K, TeSlaa T, Hoverter NP, et al. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014;33:1454–73.
Kamarajugadda S, Stemboroski L, Cai Q, Simpson NE, Nayak S, Tan M, et al. Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol. 2012;32:1893–907.
Lu CW, Lin SC, Chien CW, Lin SC, Lee CT, Lin BW, et al. Overexpression of pyruvate dehydrogenase kinase 3 increases drug resistance and early recurrence in colon cancer. Am J Pathol. 2011;179:1405–14.
Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, et al. A mitochondria-K + channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51.
Kankotia S, S PW. Dichloroacetate and cancer: new home for an orphan drug? Biochim Biophys Acta. 2014;1846:617–29.
S PW. Therapeutic targeting of the pyruvate dehydrogenase complex/pyruvate dehydrogenase kinase (PDC/PDK) axis in cancer. J Natl Cancer Inst. 2017;109:11.
Han JE, Lim PW, Na CM, Choi YS, Lee JY, Kim Y, et al. Inhibition of HIF1α and PDK Induces Cell Death of Glioblastoma Multiforme. Exp Neurobiol. 2017;26:5.
Ren YJ, Wang XH, Ji C, Guan YD, Lu XJ, Liu XR, et al. Silencing of NAC1 expression induces cancer cells oxidative stress in hypoxia and potentiates the therapeutic activity of elesclomol. Front Pharmacol. 2017;8:804.
Ilic BojanaB, Antic JadrankaA, Bankovic JovanaZ, Milicevic IvanaT, Rodic GordanaS, Ilic DusanS, et al. VHL dependent expression of redd1 and PDK3 proteins in clear-cell renal cell carcinoma. J Med Biochem. 2018;37:31–8.
Siddamalla S, Reddy TV, Govatati S, Guruvaiah P, Deenadayal M, Shivaji S, et al. Influence of tumour suppressor gene (TP53, BRCA1 and BRCA2) polymorphisms on polycystic ovary syndrome in South Indian women. Eur J Obstet Gynecol Reprod Biol. 2018;227:13–8.
Shim U, Kim HN, Lee H, Oh JY, Sung YA, K HL. Pathway analysis based on a genome-wide association study of polycystic ovary syndrome. PLoS ONE. 2015;10:e0136609.
Duffy MJ, Synnott NC, C J. Mutant p53 in breast cancer: potential as a therapeutic target and biomarker. Breast Cancer Res. 2018;170:213–9.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81500118, 61501519), the China Postdoctoral Science Foundation funded project (Project No.2016M600443), Jiangsu Province Postdoctoral Science Foundation funded project (Project No.1701184B).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cui, L., Cheng, Z., Liu, Y. et al. Overexpression of PDK2 and PDK3 reflects poor prognosis in acute myeloid leukemia. Cancer Gene Ther 27, 15–21 (2020). https://doi.org/10.1038/s41417-018-0071-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-018-0071-9
This article is cited by
-
Correlation of Glycolysis-immune-related Genes in the Follicular Microenvironment of Endometriosis Patients with ART Outcomes
Reproductive Sciences (2024)
-
A novel organic arsenic derivative MZ2 remodels metabolism and triggers mtROS-mediated apoptosis in acute myeloid leukemia
Journal of Cancer Research and Clinical Oncology (2023)
-
Establishment of a novel glycolysis-immune-related diagnosis gene signature for endometriosis by machine learning
Journal of Assisted Reproduction and Genetics (2023)
-
Retro-inversion follicle-stimulating hormone peptide-modified nanoparticles for delivery of PDK2 shRNA against chemoresistant ovarian cancer by switching glycolysis to oxidative phosphorylation
Cancer Nanotechnology (2022)
-
Hypoxia-induced circRNF13 promotes the progression and glycolysis of pancreatic cancer
Experimental & Molecular Medicine (2022)