Abstract
Background
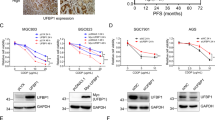
Chemoresistant cancer cells frequently exhibit a state of chronically activated endoplasmic reticulum (ER) stress. Engaged with ER stress, the unfolded protein response (UPR) is an adaptive reaction initiated by the accumulation of misfolded proteins. Protein disulfide isomerase (PDI) is a molecular chaperone known to be highly expressed in glioblastomas with acquired resistance to temozolomide (TMZ). We investigate whether therapeutic targeting of PDI provides a rationale to overcome chemoresistance.
Methods
The activity of PDI was suppressed in glioblastoma cells using a small molecule inhibitor CCF642. Either single or combination treatment with TMZ was used. We prepared nanoformulation of CCF642 loaded in albumin as a drug carrier for orthotopic tumour model.
Results
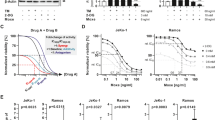
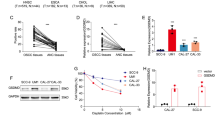
Inhibition of PDI significantly enhances the cytotoxic effect of TMZ on glioblastoma cells. More importantly, inhibition of PDI is able to sensitise glioblastoma cells that are initially resistant to TMZ treatment. Nanoformulation of CCF642 is well-tolerated and effective in suppressing tumour growth. It activates cell death-triggering UPR beyond repair and induces ER perturbations through the downregulation of PERK signalling. Combination treatment of TMZ with CCF642 significantly reduces tumour growth compared with either modality alone.
Conclusion
Our study demonstrates modulation of ER stress by targeting PDI as a promising therapeutic rationale to overcome chemoresistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data presented in this study are available from the corresponding author upon reasonable request.
References
Saunders NA, Simpson F, Thompson EW, Hill MM, Endo-Munoz L, Leggatt G, et al. Role of intratumoural heterogeneity in cancer drug resistance: molecular and clinical perspectives. EMBO Mol Med. 2012;4:675–84.
Happold C, Roth P, Wick W, Schmidt N, Florea AM, Silginer M, et al. Distinct molecular mechanisms of acquired resistance to temozolomide in glioblastoma cells. J Neurochem. 2012;122:444–55.
Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29.
Salaroglio IC, Panada E, Moiso E, Buondonno I, Provero P, Rubinstein M, et al. PERK induces resistance to cell death elicited by endoplasmic reticulum stress and chemotherapy. Mol Cancer. 2017;16:91.
Sun S, Lee D, Ho AS, Pu JK, Zhang XQ, Lee NP, et al. Inhibition of prolyl 4-hydroxylase, beta polypeptide (P4HB) attenuates temozolomide resistance in malignant glioma via the endoplasmic reticulum stress response (ERSR) pathways. Neuro Oncol. 2013;15:562–77.
Sun S, Wong TS, Zhang XQ, Pu JK, Lee NP, Day PJ, et al. Protein alterations associated with temozolomide resistance in subclones of human glioblastoma cell lines. J Neurooncol. 2012;107:89–100.
Sun S, Kiang KMY, Ho ASW, Lee D, Poon MW, Xu FF, et al. Endoplasmic reticulum chaperone prolyl 4-hydroxylase, beta polypeptide (P4HB) promotes malignant phenotypes in glioma via MAPK signaling. Oncotarget. 2017;8:71911–23.
Sun S, Kiang KM, Leung GK. Chaperone protein P4HB predicts temozolomide response and prognosis in malignant glioma. Oncol Lett. 2022;24:264.
Samanta S, Tamura S, Dubeau L, Mhawech-Fauceglia P, Miyagi Y, Kato H, et al. Expression of protein disulfide isomerase family members correlates with tumor progression and patient survival in ovarian cancer. Oncotarget. 2017;8:103543–56.
Yu SJ, Won JK, Ryu HS, Choi WM, Cho H, Cho EJ, et al. A novel prognostic factor for hepatocellular carcinoma: protein disulfide isomerase. Korean J Intern Med. 2014;29:580–7.
Kim KM, An AR, Park HS, Jang KY, Moon WS, Kang MJ, et al. Combined expression of protein disulfide isomerase and endoplasmic reticulum oxidoreductin 1-alpha is a poor prognostic marker for non-small cell lung cancer. Oncol Lett. 2018;16:5753–60.
Goplen D, Wang J, Enger PO, Tysnes BB, Terzis AJ, Laerum OD, et al. Protein disulfide isomerase expression is related to the invasive properties of malignant glioma. Cancer Res. 2006;66:9895–902.
Lovat PE, Corazzari M, Armstrong JL, Martin S, Pagliarini V, Hill D, et al. Increasing melanoma cell death using inhibitors of protein disulfide isomerases to abrogate survival responses to endoplasmic reticulum stress. Cancer Res. 2008;68:5363–9.
Eirich J, Braig S, Schyschka L, Servatius P, Hoffmann J, Hecht S, et al. A small molecule inhibits protein disulfide isomerase and triggers the chemosensitization of cancer cells. Angew Chem Int Ed Engl. 2014;53:12960–5.
Kaplan A, Gaschler MM, Dunn DE, Colligan R, Brown LM, Palmer AG 3rd, et al. Small molecule-induced oxidation of protein disulfide isomerase is neuroprotective. Proc Natl Acad Sci USA. 2015;112:E2245–52.
Vatolin S, Phillips JG, Jha BK, Govindgari S, Hu J, Grabowski D, et al. Novel protein disulfide isomerase inhibitor with anticancer activity in multiple myeloma. Cancer Res. 2016;76:3340–50.
Liu Y, Ji W, Shergalis A, Xu J, Delaney AM, Calcaterra A, et al. Activation of the unfolded protein response via inhibition of protein disulfide isomerase decreases the capacity for DNA repair to sensitize glioblastoma to radiotherapy. Cancer Res. 2019;79:2923–32.
Xu S, Liu Y, Yang K, Wang H, Shergalis A, Kyani A, et al. Inhibition of protein disulfide isomerase in glioblastoma causes marked downregulation of DNA repair and DNA damage response genes. Theranostics. 2019;9:2282–98.
Lampson LA. Monoclonal antibodies in neuro-oncology: getting past the blood-brain barrier. MAbs. 2011;3:153–60.
Zhou G, Jin X, Zhu P, Yao JU, Zhang Y, Teng L, et al. Human serum albumin nanoparticles as a novel delivery system for cabazitaxel. Anticancer Res. 2016;36:1649–56.
Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol. 2008;26:57–64.
Zong Y, Wu J, Shen K. Nanoparticle albumin-bound paclitaxel as neoadjuvant chemotherapy of breast cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:17360–72.
McBain C, Lawrie TA, Rogozinska E, Kernohan A, Robinson T, Jefferies S. Treatment options for progression or recurrence of glioblastoma: a network meta-analysis. Cochrane Database Syst Rev. 2021;5:CD013579.
Montane J, de Pablo S, Obach M, Cadavez L, Castano C, Alcarraz-Vizan G, et al. Protein disulfide isomerase ameliorates beta-cell dysfunction in pancreatic islets overexpressing human islet amyloid polypeptide. Mol Cell Endocrinol. 2016;420:57–65.
Hasipek M, Grabowski D, Guan Y, Alugubelli RR, Tiwari AD, Gu X, et al. Therapeutic targeting of protein disulfide isomerase PDIA1 in multiple myeloma. Cancers. 2021;13:2649.
Sisinni L, Pietrafesa M, Lepore S, Maddalena F, Condelli V, Esposito F, et al. Endoplasmic reticulum stress and unfolded protein response in breast cancer: the balance between apoptosis and autophagy and its role in drug resistance. Int J Mol Sci. 2019;20:857.
Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32:805–18.
Markouli M, Strepkos D, Papavassiliou AG, Piperi C. Targeting of endoplasmic reticulum (ER) stress in gliomas. Pharm Res. 2020;157:104823.
Acknowledgements
This work was supported by the Health and Medical Research Fund (HMRF), project no.08191986, of the Food and Health Bureau (Hong Kong). We would like to thank the University of Hong Kong LKS Faculty of Medicine the Centre of PanorOmic Sciences (Imaging and flow cytometry core), and the Electron Microscope Unit for their assistance and services.
Funding
This work was supported by the Health and Medical Research Fund (HMRF), project no. 08191986, of the Food and Health Bureau (Hong Kong).
Author information
Authors and Affiliations
Contributions
KK performed the experiments, wrote the original draft and involved in conceptualisation and funding acquisition. WT, JL and TL performed the experiments and provided technical support. QS and HS helped in the preparation of nanoparticles and provided technical support. NL participated in the formal data analyses. ZZ and GL supervised the entire study and provided critical review on the manuscript. All authors reviewed the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
HCS is a scientific advisor of EN Technology Limited in which he owns some equity, and also a managing director of the research centre, namely Advanced Biomedical Instrumentation Centre Limited. The works in the paper are however not directly related to the works of these two entities, as far as we know. The remaining authors declare no competing interests.
Ethics approval and consent to participate
Animal work was performed according to guidelines approved by the Committee on the Use of Live Animal for Teaching and Research, The University of Hong Kong. The use of animals and all experimental procedures were conducted in compliance to ARRIVE guidelines.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kiang, K.MY., Tang, W., Song, Q. et al. Targeting unfolded protein response using albumin-encapsulated nanoparticles attenuates temozolomide resistance in glioblastoma. Br J Cancer 128, 1955–1963 (2023). https://doi.org/10.1038/s41416-023-02225-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02225-x
This article is cited by
-
SEC23A confers ER stress resistance in gastric cancer by forming the ER stress-SEC23A-autophagy negative feedback loop
Journal of Experimental & Clinical Cancer Research (2023)