Abstract
Background
The direct comparison of molecular responses of front-line imatinib (IM) monitored at the same laboratory between children and adults with chronic phase (CP) of chronic myeloid leukaemia (CML) had not been reported. In this multicenter study, we compared the landmark molecular responses and outcomes of paediatric and adult CML-CP cohorts treated with front-line IM in whom the BCR::ABL1 transcript levels were monitored at the same accredited laboratory in Taiwan.
Methods
Between June 2004 and July 2020, 55 newly diagnosed paediatric and 782 adult CML-CP patients, with molecular diagnosis and monitoring at the same reference laboratory in Taiwan, were enrolled. The criteria of 2020 European LeukemiaNet were applied to evaluate the molecular responses.
Results
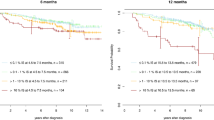
By year 5, the cumulative incidences of IS <1%, MMR, MR4.0 and MR4.5 of paediatric patients were all significantly lower than those of adult patients (58 vs 75%, 48 vs 66%, 25 vs 44%, 16 vs 34%, respectively). The 10-year progression-free survival (PFS) (90%) and overall survival (OS) (94%) of paediatric patients did not differ from those (92%) of adult patients.
Conclusions
We demonstrated the paediatric cohort had slower molecular responses to front-line IM and similar outcomes in 10-year PFS and OS in real-world practice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated and/or analysed during this study are available from the corresponding author on reasonable request.
References
Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–51.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84.
Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84.
Zhen C, Wang YL. Molecular monitoring of chronic myeloid leukemia: international standardization of BCR-ABL1 quantitation. J Mol Diagn. 2013;15:556–64.
Howlader N, Noone A, Krapcho M, Miller D, Bishop K, Altekruse S, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; 2016.
Health Promotion Administration, Ministry of Health and Welfare: CANCER REGISTRY ANNUAL REPORT, 2014-2016 TAIWAN. https://www.hpa.gov.tw/Pages/List.aspx?nodeid=119.
de la Fuente J, Baruchel A, Biondi A, de Bont E, Dresse MF, Suttorp M, et al. Managing children with chronic myeloid leukaemia (CML): recommendations for the management of CML in children and young people up to the age of 18 years. Br J Haematol. 2014;167:33–47.
Hijiya N, Schultz KR, Metzler M, Millot F, Suttorp M. Pediatric chronic myeloid leukemia is a unique disease that requires a different approach. Blood. 2016;127:392–9.
Athale U, Hijiya N, Patterson BC, Bergsagel J, Andolina JR, Bittencourt H, et al. Management of chronic myeloid leukemia in children and adolescents: recommendations from the Children’s Oncology Group CML Working Group. Pediatr Blood Cancer. 2019;66:e27827.
Hijiya N, Suttorp M. How I treat chronic myeloid leukemia in children and adolescents. Blood. 2019;133:2374–84.
Millot F, Baruchel A, Guilhot J, Petit A, Leblanc T, Bertrand Y, et al. Imatinib is effective in children with previously untreated chronic myelogenous leukemia in early chronic phase: results of the French national phase IV trial. J Clin Oncol. 2011;29:2827–32.
Giona F, Putti MC, Micalizzi C, Menna G, Moleti ML, Santoro N, et al. Long-term results of high-dose imatinib in children and adolescents with chronic myeloid leukaemia in chronic phase: the Italian experience. Br J Haematol. 2015;170:398–407.
Janeczko-Czarnecka M, Krawczuk-Rybak M, Karpinska-Derda I, Niedzwiecki M, Musiol K, Cwiklinska M, et al. Imatinib in the treatment of chronic myeloid leukemia in children and adolescents is effective and well tolerated: report of the Polish Pediatric Study Group for the Treatment of Leukemias and Lymphomas. Adv Clin Exp Med. 2018;27:91–8.
Suttorp M, Schulze P, Glauche I, Gohring G, von Neuhoff N, Metzler M, et al. Front-line imatinib treatment in children and adolescents with chronic myeloid leukemia: results from a phase III trial. Leukemia. 2018;32:1657–69.
Pemmaraju N, Kantarjian H, Shan J, Jabbour E, Quintas-Cardama A, Verstovsek S, et al. Analysis of outcomes in adolescents and young adults with chronic myelogenous leukemia treated with upfront tyrosine kinase inhibitor therapy. Haematologica. 2012;97:1029–35.
Proschmann R, Baldow C, Rothe T, Suttorp M, Thiede C, Tauer JT, et al. Response dynamics of pediatric patients with chronic myeloid leukemia on imatinib therapy. Haematologica. 2017;102:e39–e42.
Castagnetti F, Gugliotta G, Baccarani M, Breccia M, Specchia G, Levato L, et al. Differences among young adults, adults and elderly chronic myeloid leukemia patients. Ann Oncol. 2015;26:185–92.
Millot F, Guilhot J, Baruchel A, Petit A, Bertrand Y, Mazingue F, et al. Impact of early molecular response in children with chronic myeloid leukemia treated in the French Glivec phase 4 study. Blood. 2014;124:2408–10.
Marin D, Ibrahim AR, Lucas C, Gerrard G, Wang L, Szydlo RM, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232–8.
Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37.
Cross NC, White HE, Ernst T, Welden L, Dietz C, Saglio G, et al. Development and evaluation of a secondary reference panel for BCR-ABL1 quantification on the International Scale. Leukemia. 2016;30:1844–52.
Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63:789–99.
Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118:686–92.
Pfirrmann M, Baccarani M, Saussele S, Guilhot J, Cervantes F, Ossenkoppele G, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30:48–56.
Kuo M-C, Chen T-Y, Wang M-C, Yang Y, Yeh S-P, Chang C-S, et al. No Differences in outcomes between patients achieving early molecular response at 3 months and those achieving optimal response at 6 or 12 months in chronic phase of chronic myeloid leukemia treated with front-line imatinib: Taiwan CML study. Blood. 2014;124:4544.
Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917–27.
Hehlmann R, Lauseker M, Saussele S, Pfirrmann M, Krause S, Kolb HJ, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31:2398–406.
Oehler VG. First-generation vs second-generation tyrosine kinase inhibitors: which is best at diagnosis of chronic phase chronic myeloid leukemia? Hematol Am Soc Hematol Educ Program. 2020;2020:228–36.
Ganta RR, Nasaka S, Gundeti S. Impact of imatinib adherence on the cytogenetic response in pediatric chronic myeloid leukemia—chronic phase. Indian J Pediatr. 2016;83:1009–12.
Shao H, Zeng Z, Cen J, Zhang J, Bai S, Wu C, et al. The impact of early molecular response in children and adolescents with chronic myeloid leukemia treated with imatinib: a single-center study from China. Leuk Lymphoma. 2018;59:2152–8.
Lakshmaiah KC, Bhise R, Purohit S, Abraham LJ, Lokanatha D, Suresh TM, et al. Chronic myeloid leukemia in children and adolescents: results of treatment with imatinib mesylate. Leuk Lymphoma. 2012;53:2430–3.
Ganguly S, Pushpam D, Mian A, Chopra A, Gupta R, Bakhshi S. Real-world experience of imatinib in pediatric chronic phase chronic myeloid leukemia: a single-center experience from India. Clin Lymphoma Myeloma Leuk. 2020;20:e437–e44.
Madabhavi I, Patel A, Modi G, Anand A, Panchal H, Parikh S. Pediatric chronic myeloid leukemia: a single-center experience. J Cancer Res Ther. 2020;16:110–5.
Deng M, Guan X, Wen X, Xiao J, An X, Yu J. Clinical efficacy and safety of imatinib treatment in children and adolescents with chronic myeloid leukemia: a single-center experience in China. Medicine. 2020;99:e19150.
Cai Y, Liu C, Guo Y, Chen X, Zhang L, Chen Y, et al. Long-term safety and efficacy of imatinib in pediatric patients with chronic myeloid leukemia: single-center experience from China. Int J Hematol. 2021;113:413–21.
Champagne MA, Fu CH, Chang M, Chen H, Gerbing RB, Alonzo TA, et al. Higher dose imatinib for children with de novo chronic phase chronic myelogenous leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2011;57:56–62.
Branford S, Kim DDH, Apperley JF, Eide CA, Mustjoki S, Ong ST, et al. Laying the foundation for genomically-based risk assessment in chronic myeloid leukemia. Leukemia. 2019;33:1835–50.
Ernst T, Busch M, Rinke J, Ernst J, Haferlach C, Beck JF, et al. Frequent ASXL1 mutations in children and young adults with chronic myeloid leukemia. Leukemia. 2018;32:2046–9.
Roche-Lestienne C, Marceau A, Labis E, Nibourel O, Coiteux V, Guilhot J, et al. Mutation analysis of TET2, IDH1, IDH2 and ASXL1 in chronic myeloid leukemia. Leukemia. 2011;25:1661–4.
Schonfeld L, Rinke J, Hinze A, Nagel SN, Schafer V, Schenk T, et al. ASXL1 mutations predict inferior molecular response to nilotinib treatment in chronic myeloid leukemia. Leukemia. 2022;36:2242–9.
Thornley I, Perentesis JP, Davies SM, Smith FO, Champagne M, Lipton JM. Treating children with chronic myeloid leukemia in the imatinib era: a therapeutic dilemma? Med Pediatr Oncol. 2003;41:115–7.
Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl J Med. 2006;355:2408–17.
de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26:3358–63.
Hochhaus A, O’Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–61.
Pfirrmann M, Clark RE, Prejzner W, Lauseker M, Baccarani M, Saussele S, et al. The EUTOS long-term survival (ELTS) score is superior to the Sokal score for predicting survival in chronic myeloid leukemia. Leukemia. 2020;34:2138–49.
Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst. 1998;90:850–8.
Gurrea Salas D, Glauche I, Tauer JT, Thiede C, Suttorp M. Can prognostic scoring systems for chronic myeloid leukemia as established in adults be applied to pediatric patients? Ann Hematol. 2015;94:1363–71.
Millot F, Guilhot J, Suttorp M, Gunes AM, Sedlacek P, De Bont E, et al. Prognostic discrimination based on the EUTOS long-term survival score within the International Registry for Chronic Myeloid Leukemia in children and adolescents. Haematologica. 2017;102:1704–8.
Leung WY, Cheuk DK, Cheng FW, Leung AW, Chiu KH, Ho KK, et al. Outcome prediction of chronic myeloid leukemia (CML) in children. Ann Hematol. 2022;101:1677–88.
Acknowledgements
The authors would like to thank Ms. Chang-Liang Lai for her technical assistance; and Drs Fang-Liang Huang, Tsung-Yen Chang, Te-Kau Chang, Tseng-Hsi Lin, Ching-Yuan Kuo, Chih-Cheng Chen, Ming-Sun Yu, Hung-I Cheng, and Yu-Shin Hung for providing patient samples.
Funding
This work was supported by grants from Chang Gung Memorial Hospital, Taiwan (CMRPG350071 and XMRPG1A0081).
Author information
Authors and Affiliations
Contributions
H-CL and M-CK: provision of materials, data curation and writing of the manuscript. K-HW, T-YC, J-SC, M-CW, T-LL, YSY, M-CM, P-NW, J-MS, S-CW, S-HC, T-HJ, C-NC and T-CY: provision of materials and approval of the manuscript. T-HL: data analysis and approval of the manuscript. L-YS: concept design, methodology, funding acquisition, supervision, data curation and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written informed consent was obtained from the patients, parents, or guardians. Chang Gung Medical Foundation Institutional Review Board approved the study and the committee’s reference numbers are 96-0358B and 100-0927B. This study was performed in accordance with the Declaration of Helsinki.
Consent to publish
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, HC., Kuo, MC., Wu, KH. et al. Children with chronic myeloid leukaemia treated with front-line imatinib have a slower molecular response and comparable survival compared with adults: a multicenter experience in Taiwan. Br J Cancer 128, 1294–1300 (2023). https://doi.org/10.1038/s41416-023-02162-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02162-9