Abstract
Background
Procaspase-3 (PC-3) is overexpressed in multiple tumour types and procaspase-activating compound 1 (PAC-1) directly activates PC-3 and induces apoptosis in cancer cells. This report describes the first-in-human, phase I study of PAC-1 assessing maximum tolerated dose, safety, and pharmacokinetics.
Methods
Modified-Fibonacci dose-escalation 3 + 3 design was used. PAC-1 was administered orally at 7 dose levels (DL) on days 1–21 of a 28-day cycle. Dose-limiting toxicity (DLT) was assessed during the first two cycles of therapy, and pharmacokinetics analysis was conducted on days 1 and 21 of the first cycle. Neurologic and neurocognitive function (NNCF) tests were performed throughout the study.
Results
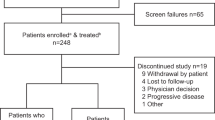
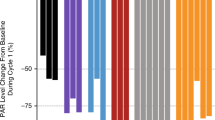
Forty-eight patients were enrolled with 33 completing ≥2 cycles of therapy and evaluable for DLT. DL 7 (750 mg/day) was established as the recommended phase 2 dose, with grade 1 and 2 neurological adverse events noted, while NNCF testing showed stable neurologic and cognitive evaluations. PAC-1’s t1/2 was 28.5 h after multi-dosing, and systemic drug exposures achieved predicted therapeutic concentrations. PAC-1 clinical activity was observed in patients with neuroendocrine tumour (NET) with 2/5 patients achieving durable partial response.
Conclusions
PAC-1 dose at 750 mg/day was recommended for phase 2 studies. Activity of PAC-1 in treatment-refractory NET warrants further investigation.
Clinical trial registration
Clinical Trials.gov: NCT02355535.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data generated in this study are available upon request from the corresponding author.
References
Dix MM, Simon GM, Cravatt BF. Global mapping of the topography and magnitude of proteolytic events in apoptosis. Cell. 2008;134:679–91.
Mahrus S, Trinidad JC, Barkan DT, Sali A, Burlingame AL, Wells JA. Global sequencing of proteolytic cleavage sites in apoptosis by specific labeling of protein N termini. Cell. 2008;134:866–76.
Boudreau MW, Peh J, Hergenrother PJ. Procaspase-3 overexpression in cancer: a paradoxical observation with therapeutic potential. ACS Chem Biol. 2019;14:2335–48.
Putt KS, Chen GW, Pearson JM, Sandhorst JS, Hoagland MS, Kwon JT, et al. Small-molecule activation of procaspase-3 to caspase-3 as a personalized anticancer strategy. Nat Chem Biol. 2006;2:543–50.
Peterson QP, Hsu DC, Goode DR, Novotny CJ, Totten RK, Hergenrother PJ. Procaspase-3 activation as an anti-cancer strategy: structure-activity relationship of procaspase-activating compound 1 (PAC-1) and its cellular co-localization with caspase-3. J Med Chem. 2009;52:5721–31.
Peterson QP, Goode DR, West DC, Ramsey KN, Lee JJY, Hergenrother PJ. PAC-1 activates procaspase-3 in vitro through relief of zinc-mediated inhibition. J Mol Biol. 2009;388:144–58.
Truong-Tran AQ, Grosser D, Ruffin RE, Murgia C, Zalewski PD. Apoptosis in the normal and inflamed airway epithelium: role of zinc in epithelial protection and procaspase-3 regulation. Biochem Pharm. 2003;66:1459–68.
West DC, Qin Y, Peterson QP, Thomas DL, Palchaudhuri R, Morrison KC, et al. Differential effects of procaspase-3 activating compounds in the induction of cancer cell death. Mol Pharm. 2012;9:1425–34.
Putinski C, Abdul-Ghani M, Stiles R, Brunette S, Dick SA, Fernando P, et al. Intrinsic-mediated caspase activation is essential for cardiomyocyte hypertrophy. Proc Natl Acad Sci USA. 2013;110:E4079–87.
Wang F, Wang L, Zhao Y, Li Y, Ping G, Xiao S, et al. A novel small-molecule activator of procaspase-3 induces apoptosis in cancer cells and reduces tumor growth in human breast, liver and gallbladder cancer xenografts. Mol Oncol. 2014;8:1640–52.
Seervi M, Sobhan PK, Mathew KA, Joseph J, Pillai PR, Santhoshkumar TR. A high-throughput image-based screen for the identification of Bax/Bak-independent caspase activators against drug-resistant cancer cells. Apoptosis. 2014;19:269–84.
Monaco G, Decrock E, Akl H, Ponsaerts R, Vervliet T, Luyten T, et al. Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl. Cell Death Differ. 2012;19:295–309.
Patel V, Balakrishnan K, Keating MJ, Wierda WG, Gandhi V. Expression of executioner procaspases and their activation by a procaspase-activating compound in chronic lymphocytic leukemia cells. Blood. 2015;125:1126–36.
Zhao HJ, Liu T, Mao X, Han S, Liang R, Hui L, et al. Fructus phyllanthi tannin fraction induces apoptosis and inhibits migration and invasion of human lung squamous carcinoma cells in vitro via MAPK/MMP pathways. Acta Pharmacol Sin. 2015;36:758–68.
Ma J, Bao G, Wang L, Li W, Xu B, Du B, et al. Design, synthesis, biological evaluation and preliminary mechanism study of novel benzothiazole derivatives bearing indole-based moiety as potent antitumor agents. Eur J Med Chem. 2015;96:173–86.
Chakkath T, Lavergne SN, Fan TM, Bunick D, Dirikolu L. Preliminary metabolism of lomustine in dogs and comparative cytotoxicity of lomustine and its major metabolites in canine cells. Vet Sci. 2014;1:159–73.
Guo WT, Wang XW, Yan YL, Li YP, Yin X, Zhang Q, et al. Suppression of epithelial-mesenchymal transition and apoptotic pathways by miR-294/302 family synergistically blocks let-7-induced silencing of self-renewal in embryonic stem cells. Cell Death Differ. 2015;22:1158–69.
Xu J, Li Z, Wang J, Chen H, Fang JY. Combined PTEN mutation and protein expression associate with overall and disease-free survival of glioblastoma patients. Transl Oncol. 2014;7:196–205.
Campbell DS, Okamoto H. Local caspase activation interacts with Slit-Robo signaling to restrict axonal arborization. J Cell Biol. 2013;203:657–72.
Zhu H, Sun G, Dong J, Fei L. The role of PRRX1 in the apoptosis of A549 cells induced by cisplatin. Am J Transl Res. 2017;9:396–402.
Dong T, Zhang Q, Hamblin MR, Wu MX. Low-level light in combination with metabolic modulators for effective therapy of injured brain. J Cereb Blood Flow Metab. 2015;35:1435–44.
Dick SA, Chang NC, Dumont NA, Bell RAV, Putinski C, Kawabe Y, et al. Caspase 3 cleavage of Pax7 inhibits self-renewal of satellite cells. Proc Natl Acad Sci USA. 2015;112:E5246–52.
Xu L, Zeng Z, Zhang W, Ren G, Ling X, Huang F, et al. RXRα ligand Z-10 induces PML-RARα cleavage and APL cell apoptosis through disrupting PML-RARα/RXRα complex in a cAMP-independent manner. Oncotarget. 2017;8:12311–22.
Namjoshi P, Showalter L, Czerniecki BJ, Koski GK. T-helper 1-type cytokines induce apoptosis and loss of HER-family oncodriver expression in murine and human breast cancer cells. Oncotarget. 2019;10:6006–20.
Auner HW, Beham-Schmid C, Dillon N, Sabbattini P. The life span of short-lived plasma cells is partly determined by a block on activation of apoptotic caspases acting in combination with endoplasmic reticulum stress. Blood. 2010;116:3445–55.
Wang H, Boussouar A, Mazelin L, Tauszig-Delamasure S, Sun Y, Goldschneider D, et al. The proto-oncogene c-Kit inhibits tumor growth by behaving as a dependence receptor. Mol Cell. 2018;72:413–25.
Yosefzon Y, Soteriou D, Feldman A, Kostic L, Koren E, Brown S, et al. Caspase-3 regulates YAP-dependent cell proliferation and organ size. Mol Cell. 2018;70:573–87.e4.
Chen Y, Sun M, Ding J, Zhu Q. SM-1, a novel PAC-1 derivative, activates procaspase-3 and causes cancer cell apoptosis. Cancer Chemother Pharm. 2016;78:643–54.
Zhai X, Bao G, Wang L, Cheng M, Zhao M, Zhao S, et al. Design, synthesis and biological evaluation of novel 4-phenoxy-6,7-disubstituted quinolines possessing (thio)semicarbazones as c-Met kinase inhibitors. Bioorg Med Chem. 2016;24:1331–45.
Ma J, Chen D, Lu K, Wang L, Han X, Zhao Y, et al. Design, synthesis, and structure-activity relationships of novel benzothiazole derivatives bearing the ortho-hydroxy N-carbamoylhydrazone moiety as potent antitumor agents. Eur J Med Chem. 2014;86:257–69.
Ma J, Zhang G, Han X, Bao G, Wang L, Zhai X. et al. Synthesis and biological evaluation of benzothiazole derivatives bearing the ortho-hydroxy-N-acylhydrazone moiety as potent antitumor agents. Arch Pharm. 2014;347:936–49.
Wang F, Wang L, Li Y, Wang N, Wang Y, Cao Q, et al. PAC-1 and its derivative WF-210 inhibit angiogenesis by inhibiting VEGF/VEGFR pathway. Eur J Pharm. 2018;821:29–38.
Botham RC, Fan TM, Im I, Borst LB, Dirikolu L, Hergenrother PJ. Dual small-molecule targeting of procaspase-3 dramatically enhances zymogen activation and anticancer activity. J Am Chem Soc. 2014;136:1312–9.
Peh J, Fan TM, Wycislo KL, Roth HS, Hergenrother PJ. The combination of vemurafenib and procaspase-3 activation is synergistic in mutant BRAF melanomas. Mol Cancer Ther. 2016;15:1859–69.
Botham RC, Roth HS, Book AP, Roady PJ, Fan TM, Hergenrother PJ. Small-molecule procaspase-3 activation sensitizes cancer to treatment with diverse chemotherapeutics. ACS Cent Sci. 2016;2:545–59.
Lucas PW, Schmit JM, Peterson QP, West DC, Hsu DC, Novotny CJ, et al. Pharmacokinetics and derivation of an anticancer dosing regimen for PAC-1, a preferential small molecule activator of procaspase-3, in healthy dogs. Invest N Drugs. 2011;29:901–11.
Joshi AD, Botham RC, Schlein LJ, Roth HS, Mangraviti A, Borodovsky A, et al. Synergistic and targeted therapy with a procaspase-3 activator and temozolomide extends survival in glioma rodent models and is feasible for the treatment of canine malignant glioma patients. Oncotarget. 2017;8:80124–38.
Schlein LJ, Fadl-Alla B, Pondenis HC, Lezmi S, Eberhart CG, LeBlanc AK, et al. Immunohistochemical characterization of procaspase-3 overexpression as a druggable target with PAC-1, a procaspase-3 activator, in canine and human brain cancers. Front Oncol. 2019;9:96.
Tonogai EJ, Huang S, Botham RC, Berry MR, Joslyn SK, Daniel GB, et al. Evaluation of a procaspase-3 activator with hydroxyurea or temozolomide against high-grade meningioma in cell culture and canine cancer patients. Neuro Oncol. 2021;23:1723–35.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
USDHHS. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2010. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03.
Spencer MPF, Folstein MF. The Mini-Mental State Examination. In: Keller PAR, Ritt LG (eds). Innovations in clinical practice: a source book. Sarasota, FL: Professional Resource Exchange, Inc.; 1985) pp 307–8.
Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13:348–58.
Brandt J, Benedict R. Hopkins Verbal Learning Test-revised. Lutz, FL: Psychological Assessment Resources, Inc.; 2001.
Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55.
Reitan R. Manual for administration of neuropsychological test batteries for adults and children. Tucson, AZ: Reitan Neuropsychology Laboratories; 1978.
Levine AJ, Miller EN, Becker JT, Selnes OA, Cohen BA. Normative data for determining significance of test-retest differences on eight common neuropsychological instruments. Clin Neuropsychol. 2004;18:373–84.
Benton AL, Hamsher KD, Siva AB. Multilingual aphasia examination. Lutz, FL: Psychological Assessment Resources, Inc; 1994.
Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11:329–38.
Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–8.
Neurocognitive Test Summary provided by the Health Services and Research Outcomes (HSRO) Subcommittee of the Radiation Therapy Oncology Group (RTOG). 2011. www.rtog.org.
Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–9.
Wefel JS, Cloughesy T, Zazzali JL, Zheng M, Prados M, Wen PY, et al. Neurocognitive function in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13:660–8.
Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–13.
Peh J, Boudreau MW, Smith HM, Hergenrother PJ. Overcoming resistance to targeted anticancer therapies through small-molecule-mediated MEK degradation. Cell Chem Biol. 2018;25:996.e4–1005.e4.
Acknowledgements
We thank James P. Zacny, PhD for his editorial support with the clinical protocol and manuscript. LC-MS/MS was performed at the Toxicology Research Laboratory, College of Medicine at the University of Illinois at Chicago. We thank the patients who participated and the Clinical Trials Office of the University of Illinois Cancer Center for providing the necessary clinical research infrastructure to execute this study.
Funding
Financial support was provided by Vanquish Oncology. Inc., the University of Illinois Cancer Center, the University of Illinois, the NIH (R01CA120439), and Engdahl Family Foundation.
Author information
Authors and Affiliations
Contributions
AZD and OCD designed the study. OCD, MH, RAP, NKV, and MJR acquired the data. OCD, MH, RAP, and NKV provided RECIST assessments. JHF did the pharmacokinetic testing. LCL did the statistical analysis. AZD, PJH, TMF, TMT, and OCD provided data analysis. AZD and OCD prepared the initial draft of the manuscript. Revisions were prepared by AZD, PJH, TMF, TMT, and OCD. All authors contributed to subsequent manuscript drafts and agreed with the submission of the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
PJH serves as Chief Scientific Officer and has equity in Vanquish Oncology, Inc. TMT serves as Chief Executive Officer and has equity in Vanquish Oncology, Inc. TMF serves as VP Preclinical Development and has equity in Vanquish Oncology, Inc. AZD reports research funding from Eli Lilly. AZD served as Chief Medical Officer for Vanquish Oncology, Inc. AZD serves as Chief Executive Officer for TTC Oncology, LLC, and Chief Medical Officer for IGF Oncology. All other authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted according to International Conference on Harmonisation of Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. The protocol was approved by the Institutional Review Board/Ethics Committee at each study location. All patients provided written informed consent before enrolment.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Danciu, O.C., Holdhoff, M., Peterson, R.A. et al. Phase I study of procaspase-activating compound-1 (PAC-1) in the treatment of advanced malignancies. Br J Cancer 128, 783–792 (2023). https://doi.org/10.1038/s41416-022-02089-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-02089-7