As good health is a lifetime issue, long-term follow up is an important part of evaluating any medical condition or treatment. This is well appreciated in epidemiologic studies where exposure to a harmful substance is often long-term, and its impact on health can appear many years after first occurrence. Examples include the classic study of Doll and Peto [1] on 34,439 male British doctors, which began in 1951 and last reported in 2004 after 50 years of follow up, in which lifelong cigarette smoking was shown to reduce life expectancy by an average of 10 years, but cessation at age 60, 50, 40 or 30 years reduced this by about 3, 6, 9, or almost the full 10 years, respectively. Other classic long-term epidemiologic studies have focussed on diet and alcohol consumption [2, 3], hormone replacement therapy [4], or have been more general and studied a wider set of risk factors, often among healthcare professionals; for example, the ACS Cancer Prevention Study [5], the Harvard-based Nurses Health Studies [6] and the Health Professionals Follow Up Study [7].
Screening trials
Treatments for clinical problems are usually directed at an immediate health issue, and obtaining data on long-term sequelae may seem less relevant and can be expensive and difficult to organise, especially in countries which do not have national repositories containing the needed information. For these reasons, long-term follow up is often not pursued. Long-term consequences are much more relevant for screening interventions and prophylactic treatments aimed at preventing future cancer, such as the value of human papilloma virus (HPV) vaccination, or the importance of screening for breast and colorectal cancer. For example, for breast cancer screening with short term follow up many cancers were found which did not appear to require treatment, leading to the belief that up to 50% of the screen detected cancers were over-diagnosis of indolent cancers that would not be clinically detected in the woman’s lifetime [8], and this was a drawback of breast screening. However, with longer follow up [9] these cancers were found to be earlier diagnosis of clinically relevant disease, and it was estimated that of the total number of screen detected cancers in the screened population, only 9.5% overall were over-diagnosed, and this was reduced to 3.7% after adjustment for self-selection. Thus, long-term follow up, even after screening has ended, is necessary to accurately assess over-diagnosis. Also, additional procedures and late side-effects need to be recorded and analysed.
Early studies clearly demonstrated the value of the cytological Papanicolaou smear test in identifying cervical lesions at a treatable stage and reducing mortality. This has been well established for many decades, and the evidence was so clear that randomised clinical trials were not needed for confirmation [10], although several long-term cohort studies have demonstrated the benefits of screening in reducing cervix cancer mortality [11]. More recently, tests have been developed for HPV, which causes cervix cancer, and trials have been conducted in which all women receive both HPV and Pap tests, and those positive for either test are referred to colposcopy to directly compare the two tests in the same woman [12]. These trials have clearly shown that HPV tests detect more cervical lesions than cytology in a range of countries and settings. Four randomised European trials with extended individual patient follow up in 176,464 women have been conducted to compare the impact of an HPV test vs. the Pap test on subsequent cancer rates. After a 6.5-year median follow up, these trials have demonstrated that HPV testing reduces cancer incidence by a further 40% overall compared with cytology, and by 70% when the HPV test was negative [13]. Longer follow up of these studies is needed to demonstrate that this reduction in cancer incidence translates into a reduction in cervix cancer mortality. However, a large four-arm cluster randomised trial of 131,746 women in rural India has also reported substantial increases in detection of precursor lesions, and, after a 20-year follow up, a significant reduction in cervix cancer deaths (34 vs. 64), which was not seen with Pap cytology or visual inspection (VIA) was also demonstrated [14]. A range of trials have also been conducted that look at the duration of protection following a negative HPV test, and after a 6-year follow up, Dillner et al. [15] have demonstrated a roughly fourfold reduction in high-grade precursor lesions and cancer (CIN3+) after a negative HPV test compared with a negative cytology test. These findings have been confirmed in a recent systematic review [16]. Several studies have also shown that HPV testing can be done on a vaginal self-sample or a urine sample, and achieves similar sensitivity for high-grade CIN, although specificity was somewhat lower [17]. This approach promises to increase acceptance of cervical screening for women, especially for those who feel uncomfortable having their sample taken by a clinician. Additional follow up of these studies will be necessary to determine which self-sampling device is best and the most appropriate screening interval when self-sampling is performed.
The Flexi-Sig screening trial of once-in-a-lifetime sigmoidoscopy for preventing colorectal cancer randomised 170,034 individuals, aged 55–64 years, in a 2:1 ratio to a single sigmoidoscopy or no screening. After 17 years of follow up it has demonstrated that the higher detection of precursor lesions found with screening was followed by a 26% reduction overall in colorectal cancer incidence, and a 30% reduction in mortality, based on 41% and 46% reductions in distal cancer incidence and mortality respectively [18]. When restricted to those actually receiving screening, larger reductions were seen: 56% and 66%, respectively [18]. Similar results have been seen for the colorectal component of the PLCO trial [19] and elsewhere [20].
Prostate-specific antigen (PSA) screening for prostate cancer is a controversial subject, where even long-term follow up of a large number of trials has not resolved the major issues. While all investigators acknowledge that screening has led to a substantial amount of over-diagnosis and over-treatment, the extent of a reduction in prostate cancer mortality, if any, remains controversial. The large European ERSPC trial has reported a 20% reduction in mortality after a maximum of 16 years of follow up in 182,160 men [21] with increasing benefit occurring with longer follow up, and the number needed to treat to prevent one prostate cancer death falling from 48 after 9 years of follow up [22] to 27 after 13 years [23] and 18 after 16 years [21]. Support for this method of screening as also been expressed [24] from North American investigators [24]. However, other studies have not been so positive, with conclusions of little or no mortality benefit in large studies and concerns about side-effects associated with treatment of indolent disease. Notable among these are the PLCO trial, with 13-year follow up in 76,685 men [25], an individual patient overview of 5 trials of 727,718 men with a 10-year follow up [26], and a large overview view of 1,904,950 patients in 63 studies [27]. These studies compared an invitation for screening with usual care, and were conducted mostly in North America, for which there was likely to be a substantial amount of opportunistic screening in the control arm. These results indicate that study size and length of follow up are not the only relevant factors. Monitoring and follow up procedures also need to be examined. Selection of who needs screening, an efficient triage algorithm to determine who to biopsy following a positive screening test, and avoidance of over-treatment of likely indolent lesions are also areas that merit further research.
Major trials have also been conducted for ovarian cancer screening, with mixed results. The most recently reported was the UKCTOCS trial, which randomised women to an annual CA125 blood test (N = 50,640), annual ultrasound (N = 50,639), or no screening (N = 101,359). After a median follow up of 11.1 years, a much higher proportion of low-volume ovarian and peritoneal cancers was found in the CA125 group vs. controls (40% vs. 26%, P < 0.0001), along with a non-significant 16% lower ovarian cancer mortality (P = 0.23) [28], leading the investigators to continue blinded follow up for mortality. After a 16.3-year median follow up, the difference in stage distribution was maintained, but no relative improvement in ovarian cancer mortality was seen in either screened arm [29] (hazard ratio for CA125 group = 0.96 (0.83–1.10), P = 0.52), leading to the conclusion that the stage shift did not translate into a mortality gain.
Screening for lung cancer provides another example of the need to evaluate cause specific mortality and not just rely on the detection of better risk cancers. An early major trial of screening by chest X-ray and sputum cytology in 9211 male smokers was conducted by the Mayo Clinic from 1971–83, and early findings based on screen detected cancer indicated that they were more likely to be resectable, postsurgical Stage I or II, and associated with better 5-year survival [30]. However, after 20.5 years of follow up, non-significantly more lung cancer deaths were seen in the screened group (337 vs. 303, relative risk 1.13) [31], indicating a lead time bias in screen cases detected that did not translate into a mortality benefit. While chest X-ray was not effective, subsequent trials using more sensitive CT scans have been shown to reduce mortality [32,33,34] and are now widely used in smokers and other high-risk individuals.
Prevention trials
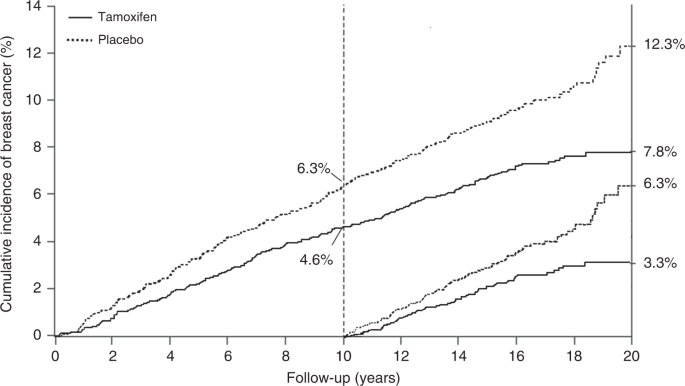
Prevention trials are an example of research intermediate between epidemiologic studies and therapeutic treatment trials. Many of these studies have been done without randomisation, and in some cases this is appropriate. However, the most informative prevention trials have been randomised, and the extra information provided by this can be very valuable. As breast cancer is the commonest cancer in women in most countries, and is known to be related to hormone levels, it is an obvious candidate for preventive trials. The first major trials examined the role of tamoxifen as a preventive medicine. It was already accepted as an effective adjuvant treatment for reduction in progression of oestrogen receptor-positive breast cancer, and in those trials a reduction was seen in new contralateral tumours, which provided a strong basis for believing it could be effective for primary prevention. Four major trials have been undertaken: the largest was a North American trial known as P-1 [35], which recruited 13,388 high-risk women, but sadly was curtailed after 7 years median follow up, so no long-term information is available. An Italian trial in 5408 hysterectomised women [36], and the IBIS-I trial, which recruited 7154 women [37] and which was preceded by the Marsden ‘pilot study’ of 2494 high-risk women [38] have also been conducted. Follow up was 11 years in the Italian trial and 20 years in the Marsden trial. Long-term follow up is continuing in the IBIS-I trial, with a 16.0-year median follow up at last publication. Long-term follow up data in that trial proved very informative, and demonstrated that 5 years of tamoxifen in high-risk women without breast cancer could prevent almost 29% of cancers over a 20-year follow up period, and the preventive effect was virtually identical in the first and second 10-year follow up periods, suggesting that the preventive effect of 5 years of tamoxifen could last a lifetime (Fig. 1). The number needed to treat to prevent one cancer was reduced from 59 after 10 years of follow up to 22 after 20 years.
Trials of raloxifene, another selective oestrogen receptor modulator like tamoxifen, have also evaluated its effect on breast cancer occurrence. The initial MORE trial was aimed at reducing fracture rates in 7705 osteoporotic women, and after 4 years of treatment it was extended for another 4 years in the CORE trial, which combined the two active treatment arms into a simple active arm at the lower dose, and retained the placebo arm. After 8 years of follow up in the combined trials a large 66% reduction in new breast cancer was seen, with an even larger 76% reduction in oestrogen receptor-positive cancer [39]. This led to the STAR trial, which compared raloxifene with tamoxifen in women at high risk of breast cancer, and after an 81-month median follow up it was found to be 24% less effective than tamoxifen, but had fewer side-effects [40]. The reasons for its lower efficacy remain unclear, but it might be related to the fact that women in MORE/CORE were osteoporotic.
Two trials have looked at the role of aromatase inhibitors in breast cancer prevention, again supported by strong findings, both for preventing recurrence and new cancers in the adjuvant setting, where they were more effective than tamoxifen [41]. The North American trial known as MAP.3 [42] showed a very large 65% reduction in invasive cancers in the first 3 years of follow up, but unfortunately follow up was also curtailed after 35 months median follow up, before long-term data could be obtained. The IBIS-II trial comparing anastrozole with placebo in 3864 high-risk postmenopausal women has now reported, with a median follow up of 10.9 years [43]. It found that reductions on new cancers continued after the 5-year active treatment period, although they were non-significantly smaller than those seen during treatment (49% overall, 61% years 0–5, 36% subsequently). Further follow up is planned, and is necessary to see if a continued preventive effect will be seen in the extended long-term follow up period, as with tamoxifen. This clearly has a major impact on number needed to treat to prevent one cancer, and will be important for determining optimal use of aromatase inhibitors for prevention.
Vaccine trials provide another good example of the need for long-term follow up. Early trials showed clearly that vaccination against HPV is effective in preventing HPV infection [44, 45] and longer term follow up of these trials has now demonstrated that this protection against infection carries forward to reduce the incidence of precursor CIN lesions [46, 47], but further follow up is needed to see if the expected reduction in cervical cancer incidence and mortality can also be achieved.
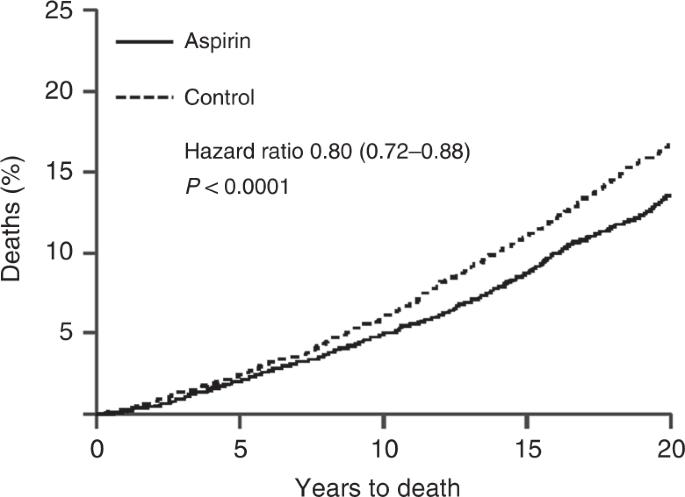
Another somewhat serendipitous set of trials has also been conducted for low-dose aspirin. These trials, of at least 4 years of aspirin or control use, were originally designed to look at its impact on cardiovascular disease, and only short term follow up, typically for less than 5 years, was reported for this. However, Peter Rothwell and colleagues [48] resurrected these 8 trials, involving 25,570 patients, and obtained information on deaths and cancers for up to 20 years of follow up, which led to the discovery of important benefits for cancer prevention, primarily for colorectal, stomach and oesophageal cancers. Curiously, very little effect on cancer was seen in the first 5 years of follow up, but large effects were seen subsequently (Fig. 2), leading to an ~20% reduction in cancer deaths overall, a 34% reduction in the post 5-year-period for all cancer deaths, and a 54% reduction in gastrointestinal cancers, which was still diverging after 20 years of follow up. The role of long-term follow up was vital in discovering this pronounced preventive activity. The clearest evidence was for a reduction in colorectal cancer, but gastric and oesophageal cancer were also substantially reduced. These results have now been validated in several non-randomised epidemiologic studies [49, 50], and are summarised in Table 1 [51]. A current challenge for understanding the role of aspirin for cancer prevention comes from the results of two recent reports on studies with short follow up [52, 53]. Given what was known from cardiovascular studies, it should have been anticipated that no benefit would be seen in the first 5 years, and only the side-effects would be apparent. This is what has happened, and has been misinterpreted by many to conclude that aspirin has no effect on cancer when, in fact, no useful efficacy information has yet to be obtained from these studies [54].
Twenty year impact of daily aspirin on deaths from all solid tumours (Rothwell et al. [48]).
The Prostate Cancer Prevention Trial (PCPT) compared the anti-androgen finasteride vs. placebo in 18,882 low to average risk men aged 55 years or over [55]. After a 7-year follow up, the trial reported a 24.8% reduction in prostate cancer cases overall (803 vs. 1147, P < 0.0001), but high-grade tumours (Gleason grade ≥7) were more common in the treated group (280 vs. 337, P = 0.005). This has led to much debate, but after further follow up for a median of 18 years, no excess in prostate cancer deaths has been observed [56]. It has been suggested that the reason for the increased detection of high-grade tumours was due to a reduction in prostate size associated with finasteride treatment, which led to more accurate biopsies being taken. This in turn may have altered the assessment of Gleason grade, again reinforcing the need for long-term follow up to discover the full impact of a clinical intervention.
Treatment trials
Long-term follow up often needs to be seen in a different context in treatment trials, as often the norm is for some form of treatment where there is considerable evidence for a short term benefit, and the ethics and safety of giving no or less treatment is a bigger concern. Thus, the main question is whether a long-term benefit exists when short-term efficacy is well established, and if it outweighs any late adverse effects.
An early example of the importance of long-term follow up in treatment trials comes from a UKCCR overview of the use of radiotherapy in early breast cancer. This overview of four early trials, initiated between 1949 and 1974, included 4148 deaths in 7842 women. No impact on deaths was seen in the first 10 years of follow up, but it was found that the regimens used at that time led to an increase in cardiac deaths for left-sided breast cancer after 10 years of follow up [57, 58]. As a result of these findings, newer radiotherapy regimens have been developed and an Early Breast Cancer Trials Coordinating Group (EBCTCG) overview has demonstrated that these regimens have led to reductions in recurrences and deaths from breast cancer, and with minimal impact on non-breast cancer deaths [59], and minimal cardiac toxicity [60, 61].
Another area where long-term follow up has provided important information has been for the use of adjuvant endocrine therapy in early breast cancer. Notable in this area are the overviews conducted for tamoxifen by the EBCTCG. Among other things, they showed that in 10,645 oestrogen receptor-positive tumours, 5 years of tamoxifen led to a 50% reduction in recurrence in the first 5 years of follow up, a further 30% reduction in years 5–9, but no further effect in years 10–14 [62]. This is in contrast to the prevention trials, where 5 years of tamoxifen prevented new breast cancers for at least 20 years [33]. For breast cancer mortality, a one-third reduction was seen in the overview, a reduction sustained for at least 15 years. In contrast to the recurrence data, mortality reductions were as strong in years 10–14 after treatment as in earlier years (Relative Risk = 0.71, 0.66 and 0.68 in each 5-year period). Tamoxifen had little effect on deaths from other causes, so that there was a substantial effect on overall mortality. It was also shown that tamoxifen had little effect on breast cancer recurrence or mortality for the 5984 oestrogen receptor poor/negative breast cancers [62].
Several large trials of aromatase inhibitors vs. tamoxifen for 5 years as adjuvant therapy in postmenopausal women have been conducted. In addition to straight two-arm comparisons, some have also compared trials which switch between aromatase inhibitors and tamoxifen after 2–3 years of initial treatment [63]. This analysis showed a larger effect for an aromatase inhibitor during its use, but not afterwards. A mortality benefit was also seen. Only one trial (ATAC) has reported even moderately long-term follow up, which has now been extended to a median of 10 years [64]. Serious side-effects were lower with anastrozole than tamoxifen.
Improvements in progression rates do not always translate into disease mortality reductions. One trial of radiotherapy for prostate cancer [65] reported improvements in biochemical progression-free survival with dose escalated radiotherapy vs. a conventional dose, which have been maintained for a median of 10 years, but this has not translated into improvements in overall survival. An overview of three available subsequent radiotherapy trials with a median follow up between 60 and 78 months was unable to establish a difference in event free survival between upfront or early salvage radiotherapy, and noted that the latter produced fewer side-effects [66]. Longer follow up, or a larger trial may be needed to see if there is an effect.
Longer term toxicity issues can also negate any improvements seen in progression markers. Mauch et al. [67] noted the excess mortality from cardiovascular disease compared with the general population in both arms of a trial of radiotherapy vs. radiotherapy and chemotherapy in Hodgkin’s disease. In an overview of 8 trials of more vs. less radiotherapy in 1974 patients, and 13 trials in 1688 patients of adding chemotherapy to radiotherapy, Specht et al. [68] reported a one-third reduction in recurrence with added radiotherapy, and a halving with added chemotherapy. However, after 10 years of follow up, no significant effect was seen on mortality, suggesting that less intensive primary treatment—particularly a reduction in radiotherapy field—may have achieved the same results. Endpoints that are surrogates for disease-specific survival, such as recurrence, metastatic spread, or increases in tumour size or grade can be indicators of better survival, but as noted above, these do not always translate into reduced disease-specific mortality [69].
Because of the longer number of years at risk after cancer treatment, long-term follow up is especially important for childhood and adolescent cancers. A range of different issues need to be addressed, including effects on mental development and childbearing. Partly because of the heterogeneity of these cancers, this is rarely done within a clinical trial, but efforts to establish registries to create large cohorts to document late side-effects are now being made [70,71,72]. This is only a start, and more resources and effort are needed to develop this area.
Not all treatment questions can be answered by a single long-term follow up analysis. Factors other than follow up duration include sample size, choice of study endpoints, aggressiveness of applying salvage therapies, and subgroups with differing prognoses [73]. Adequate sample size is essential and can be determined by standard power calculations. A common question is whether reducing disease recurrence or progression translates into an improvement in survival. Often, overall survival is used for the latter. While this clearly has value as a bottom line, for diseases in which only a small to moderate number of patients actually die from the disease under study, such as early breast or prostate cancer, a substantial loss of power can occur due to deaths from other causes for which no treatment effect is anticipated and deaths from the disease under study can be a more powerful and useful measure of efficacy [74]. Differences in other causes of death that might be due to treatment are important, but are usually specific to a particular site, and often are not captured in an ‘all other cause’ mortality assessment.
Another issue is comparing up-front treatment with salvage therapy, often studied for the use of radiotherapy. Here the threshold for using salvage therapy can be important. For example, in intermediate-risk or high-risk, localised or locally advanced prostate cancer, Vale et al. [66] found salvage therapy to be equally effective, and that it reduced the number of men with size effects.
In addition, treatments may be more or less effective in different subgroups. It is clearly clinically important to identify any heterogeneity in response, but the reporting of apparent subgroup differences is one of the most common errors in analysing clinical trial results [75, 76]. Often numerous subgroups are examined, and the chance that one is truly significantly different at, say, a 5% level, is much less than the 95% suggested by this nominal two-sided P-value, due to the multiple comparisons being made. A Bonferroni or similar correction should be made. This requires specifying the number of subgroups to be investigated, and often can be problematic. Also any difference should be based on a significant interaction between a subgroup and the remaining trial population, rather than simply a significant effect in a subgroup [77].
In other reports, randomised studies are mixed with observational studies to increase sample size, but the potential for biased allocation in the observational studies still exists [73], although it may be less than for short term follow up.
Summary
Clinically important findings can arise several years after treatment is completed, and often after formal follow up is stopped. An early analysis can give a distorted view of a treatment’s value. Especially in the prevention setting, unfavourable side-effects often occur early, and well before any benefits become apparent. In addition, favourable early effects on, e.g., recurrence, may or may not be maintained in the longer term, and may or may not lead to reductions in disease-specific mortality. Late treatment-related side-effects can also be uncovered, which can be particularly important when the prognosis is good. Many countries have national databases that can usually provide cause specific mortality, although recurrence and side effect data are less common. Linking basic long-term data to earlier clinical records can lead to valuable additional findings for a randomised clinical trial, and can provide important new findings that are more reliable than non-randomised comparisons.
Data availability
No new data was created or analysed in this report
References
Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328:1519. https://doi.org/10.1136/bmj.38142.554479.AE. Epub 2004 Jun 22. PMID: 15213107; PMCID: PMC437139.
Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–24. https://doi.org/10.1079/PHN2002394. PMID: 12639222.
Ubago-Guisado E, Rodríguez-Barranco M, Ching-López A, Petrova D, Molina-Montes E, Amiano P, et al. Evidence update on the relationship between diet and the most common cancers from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: a systematic review. Nutrients. 2021;13:3582. https://doi.org/10.3390/nu13103582. PMID: 34684583; PMCID: PMC8540388.
Beral V, Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–27. https://doi.org/10.1016/s0140-6736(03)14065-2. Erratum in: Lancet. 2003 Oct 4;362:1160. PMID: 12927427.
Jacobs EJ, Newton CC, Gapstur SM, Thun MJ. Daily aspirin use and cancer mortality in a large US cohort. J Natl Cancer Inst 2012;104:1208–17. https://doi.org/10.1093/jnci/djs318. Epub 2012 Aug 10. PMID: 22888140.
Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–96. https://doi.org/10.1038/nrc1608. PMID: 15864280.
Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. https://doi.org/10.1056/NEJMoa1014296. PMID: 21696306; PMCID: PMC3151731.
Beckmann KR, Lynch JW, Hiller JE, Farshid G, Houssami N, Duffy SW, et al. A novel case-control design to estimate the extent of over-diagnosis of breast cancer due to organised population-based mammography screening. Int J Cancer 2015;136:1411–21. https://doi.org/10.1002/ijc.29124. Epub 2014 Aug 18. PMID: 25098753.
Blyuss O, Dibden A, Massat NJ, Parmar D, Cuzick J, Duffy SW, et al. A case-control study to evaluate the impact of the breast screening programme on breast cancer incidence in England. Cancer Med. 2022 Jul. https://doi.org/10.1002/cam4.5004. Epub ahead of print. PMID: 35851849.
Tambouret RH. The evolution of the Papanicolaou smear. Clin Obstet Gynecol. 2013;56:3–9. https://doi.org/10.1097/GRF.0b013e318282b982. PMID: 23314726.
Jansen EEL, Zielonke N, Gini A, Anttila A, Segnan N, Vokó Z, et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: a systematic review. Eur J Cancer 2020;127:207–23. https://doi.org/10.1016/j.ejca.2019.12.013. Epub 2020 Jan 21. PMID: 31980322.
Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–101. https://doi.org/10.1002/ijc.21955. PMID: 16586444.
Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–32. https://doi.org/10.1016/S0140-6736:62218-7. Epub 2013 Nov 3. Erratum in: Lancet. 2015 Oct 10;386(10002):1446. PMID: 24192252.
Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. N. Engl J Med. 2009;360:1385–94. https://doi.org/10.1056/NEJMoa0808516. PMID: 19339719.
Dillner J, Rebolj M, Birembaut P, Petry KU, Szarewski A, Munk C, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008;337:a1754. https://doi.org/10.1136/bmj.a1754. PMID: 18852164; PMCID: PMC2658827.
Melnikow J, Henderson JT, Burda BU, Senger CA, Durbin S, Weyrich MS. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2018;320:687–705. https://doi.org/10.1001/jama.2018.10400. PMID: 30140883.
Arbyn M, Smith SB, Temin S, Sultana F, Castle P, Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ 2018;363:k4823. https://doi.org/10.1136/bmj.k4823. PMID: 30518635; PMCID: PMC6278587.
Atkin W, Wooldrage K, Parkin DM, Kralj-Hans I, MacRae E, Shah U, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet. 2017;389:1299–311. https://doi.org/10.1016/S0140-6736(17)30396-3. Epub 2017 Feb 22. PMID: 28236467; PMCID: PMC6168937.
Miller EA, Pinsky PF, Schoen RE, Prorok PC, Church TR. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: long-term follow-up of the randomised US PLCO cancer screening trial. Lancet Gastroenterol Hepatol. 2019;4:101–10. https://doi.org/10.1016/S2468-1253(18)30358-3. Epub 2018 Nov 29. PMID: 30502933; PMCID: PMC6335177.
Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348:g2467. https://doi.org/10.1136/bmj.g2467. PMID: 24922745; PMCID: PMC3980789.
Hugosson J, Roobol MJ, Månsson M, Tammela TLJ, Zappa M, Nelen V, et al. A 16-yr follow-up of the European Randomized Study of screening for prostate cancer. Eur Urol. 2019;76:43–51. https://doi.org/10.1016/j.eururo.2019.02.009. Epub 2019 Feb 26. PMID: 30824296; PMCID: PMC7513694.
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. https://doi.org/10.1056/NEJMoa0810084. Epub 2009 Mar 18. PMID: 19297566.
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–35. https://doi.org/10.1016/S0140-6736(14)60525-0. Epub 2014 Aug 6. PMID: 25108889; PMCID: PMC4427906.
Catalona WJ. Prostate cancer screening. Med Clin North Am. 2018;102:199–214. https://doi.org/10.1016/j.mcna.2017.11.001. PMID: 29406053; PMCID: PMC5935113.
Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–32. https://doi.org/10.1093/jnci/djr500. Epub 2012 Jan 6. PMID: 22228146; PMCID: PMC3260132.
Ilic D, Djulbegovic M, Jung JH, Hwang EC, Zhou Q, Cleves A, et al. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ. 2018;362:k3519. https://doi.org/10.1136/bmj.k3519. PMID: 30185521; PMCID: PMC6283370.
Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J. Prostate-specific antigen-based screening for prostate cancer: evidence report and systematic review for the US preventive services task force. JAMA. 2018;319:1914–31. https://doi.org/10.1001/jama.2018.3712. PMID: 29801018.
Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387:945–56. https://doi.org/10.1016/S0140-6736(15)01224-6. Epub 2015 Dec 17. Erratum in: Lancet. 2016 Mar 5;387(10022):944. Erratum in: Lancet. 2016 Mar 5;387(10022):944. PMID: 26707054; PMCID: PMC4779792.
Menon U, Gentry-Maharaj A, Burnell M, Singh N, Ryan A, Karpinskyj C, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2021;397:2182–93. https://doi.org/10.1016/S0140-6736(21)00731-5. Epub 2021 May 12. PMID: 33991479; PMCID: PMC8192829.
Fontana RS, Sanderson DR, Taylor WF, Woolner LB, Miller WE, Muhm JR, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Mayo Clinic study. Am Rev Respir Dis. 1984;130:561–5. https://doi.org/10.1164/arrd.1984.130.4.561. PMID: 6091507.
Marcus PM, Bergstralh EJ, Fagerstrom RM, Williams DE, Fontana R, Taylor WF, et al. Lung cancer mortality in the Mayo Lung Project: impact of extended follow-up. J Natl Cancer Inst. 2000;92:1308–16. https://doi.org/10.1093/jnci/92.16.1308. PMID: 10944552.
National Lung Screening Trial Research Team. Lung cancer incidence and mortality with extended follow-up in the National Lung Screening Trial. J Thorac Oncol. 2019;14:1732–42. https://doi.org/10.1016/j.jtho.2019.05.044. Epub 2019 Jun 28. PMID: 31260833; PMCID: PMC6764895.
de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–13. https://doi.org/10.1056/NEJMoa1911793. Epub 2020 Jan 29. PMID: 31995683.
Jonas DE, Reuland DS, Reddy SM, Nagle M, Clark SD, Weber RP, et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US preventive services task force. JAMA. 2021;325:971–87. https://doi.org/10.1001/jama.2021.0377. PMID: 33687468.
Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. https://doi.org/10.1093/jnci/dji372. PMID: 16288118.
Veronesi U, Maisonneuve P, Rotmensz N, Bonanni B, Boyle P, Viale G, et al. Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst. 2007;99:727–37. https://doi.org/10.1093/jnci/djk154. PMID: 17470740.
Cuzick J, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16:67–75. https://doi.org/10.1016/S1470-2045(14)71171-4. Epub 2014 Dec 11. PMID: 25497694; PMCID: PMC4772450.
Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–90. https://doi.org/10.1093/jnci/djk050. PMID: 17312305.
Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–61. https://doi.org/10.1093/jnci/djh319. PMID: 15572757.
Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Philos). 2010;3:696–706. https://doi.org/10.1158/1940-6207.CAPR-10-0076. Epub 2010 Apr 19. PMID: 20404000; PMCID: PMC2935331.
Cuzick J. Aromatase inhibitors for breast cancer prevention. J Clin Oncol. 2005;23:1636–43. https://doi.org/10.1200/JCO.2005.11.027. PMID: 15755971.
Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011;364:2381–91. https://doi.org/10.1056/NEJMoa1103507. Epub 2011 Jun 4. Erratum in: N Engl J Med. 2011 Oct 6;365(14):1361. PMID: 21639806.
Cuzick J, Sestak I, Forbes JF, Dowsett M, Cawthorn S, Mansel RE, et al. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395:117–22. https://doi.org/10.1016/S0140-6736(19)32955-1. Epub 2019 Dec 12. Erratum in: Lancet. 2020 Feb 15;395(10223):496. Erratum in: Lancet. 2021 Feb 27;397(10276):796. PMID: 31839281; PMCID: PMC6961114.
Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–51. https://doi.org/10.1056/NEJMoa020586. PMID: 12444178.
Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. https://doi.org/10.1016/S0140-6736(07)60946-5. Erratum in: Lancet. 2007 Oct 20;370(9596):1414. PMID: 17602732.
Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377:2085–92. https://doi.org/10.1016/S0140-6736(11)60551-5. PMID: 21684381.
Falcaro M, Castañon A, Ndlela B, Checchi M, Soldan K, Lopez-Bernal J, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet. 2021;398:2084–92. https://doi.org/10.1016/S0140-6736(21)02178-4. Epub 2021 Nov 3. PMID: 34741816.
Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. https://doi.org/10.1016/S0140-6736(10)62110-1. Epub 2010 Dec 6. PMID: 21144578.
Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol. 2012;23:1403–15. https://doi.org/10.1093/annonc/mds113. Epub 2012 Apr 19. PMID: 22517822.
Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–27. https://doi.org/10.1016/S1470-2045(12)70112-2. Epub 2012 Mar 21. PMID: 22440112.
Cuzick J, Thorat MA, Bosetti C, Brown PH, Burn J, Cook NR, et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. 2015;26:47–57. https://doi.org/10.1093/annonc/mdu225. Epub 2014 Aug 5. PMID: 25096604; PMCID: PMC4269341.
ASCEND Study Collaborative Group, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529–39. https://doi.org/10.1056/NEJMoa1804988. Epub 2018 Aug 26. PMID: 30146931.
McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519–28. https://doi.org/10.1056/NEJMoa1803955. Epub 2018 Sep 16. PMID: 30221595; PMCID: PMC6433466.
Chan AT. Aspirin and the USPSTF-what about cancer? JAMA Oncol. 2022. https://doi.org/10.1001/jamaoncol.2022.2967. Epub ahead of print. PMID: 35900749.
Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349:215–24. https://doi.org/10.1056/NEJMoa030660. Epub 2003 Jun 24. PMID: 12824459.
Thompson IM Jr, Goodman PJ, Tangen CM, Parnes HL, Minasian LM, Godley PA, et al. Long-term survival of participants in the prostate cancer prevention trial. N Engl J Med. 2013;369:603–10. https://doi.org/10.1056/NEJMoa1215932. PMID: 23944298; PMCID: PMC4141537.
Cuzick J, Stewart H, Peto R, Baum M, Fisher B, Host H, et al. Overview of randomized trials of postoperative adjuvant radiotherapy in breast cancer. Cancer Treat Rep. 1987;71:15–29. PMID: 2856861.
Cuzick J, Stewart H, Rutqvist L, Houghton J, Edwards R, Redmond C, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447–53. https://doi.org/10.1200/JCO.1994.12.3.447. PMID: 8120544.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–16. https://doi.org/10.1016/S0140-6736(11)61629-2. Epub 2011 Oct 19. PMID: 22019144; PMCID: PMC3254252.
Taylor CW, Kirby AM. Cardiac side-effects from breast cancer radiotherapy. Clin Oncol (R Coll Radio). 2015;27:621–9. https://doi.org/10.1016/j.clon.2015.06.007. Epub 2015 Jun 28. PMID: 26133462.
Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97:419–24. https://doi.org/10.1093/jnci/dji067. PMID: 15770005; PMCID: PMC1853253.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84. https://doi.org/10.1016/S0140-6736(11)60993-8. Epub 2011 Jul 28. PMID: 21802721; PMCID: PMC3163848.
Regan MM, Neven P, Giobbie-Hurder A, Goldhirsch A, Ejlertsen B, Mauriac L, et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8·1 years median follow-up. Lancet Oncol. 2011;12:1101–8. https://doi.org/10.1016/S1470-2045(11)70270-4. Epub 2011 Oct 20. PMID: 22018631; PMCID: PMC3235950.
Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–41. https://doi.org/10.1016/S1470-2045(10)70257-6. Epub 2010 Nov 17. PMID: 21087898.
Dearnaley DP, Jovic G, Syndikus I, Khoo V, Cowan RA, Graham JD, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464–73. https://doi.org/10.1016/S1470-2045(14)70040-3. Epub 2014 Feb 26. PMID: 24581940.
Vale CL, Fisher D, Kneebone A, Parker C, Pearse M, Richaud P, et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet. 2020;396:1422–31. https://doi.org/10.1016/S0140-6736(20)31952-8. Epub 2020 Sep 28. PMID: 33002431; PMCID: PMC7611137.
Mauch PM, Kalish LA, Marcus KC, Shulman LN, Krill E, Tarbell NJ, et al. Long-term survival in Hodgkin’s disease relative impact of mortality, second tumors, infection, and cardiovascular disease. Cancer J Sci Am. 1995;1:33–42. PMID: 9166452.
Specht L, Gray RG, Clarke MJ, Peto R. Influence of more extensive radiotherapy and adjuvant chemotherapy on long-term outcome of early-stage Hodgkin’s disease: a meta-analysis of 23 randomized trials involving 3,888 patients. International Hodgkin’s Disease Collaborative Group. J Clin Oncol. 1998;16:830–43. https://doi.org/10.1200/JCO.1998.16.3.830. PMID: 9508163.
Buyse M, Saad ED, Burzykowski T, Regan MM, Sweeney CS. Surrogacy beyond prognosis: the importance of “Trial-Level” surrogacy. Oncologist. 2022;27:266–71. https://doi.org/10.1093/oncolo/oyac006. PMID: 35380717; PMCID: PMC8982389.
Suh E, Stratton KL, Leisenring WM, Nathan PC, Ford JS, Freyer DR, et al. Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: a retrospective cohort analysis from the Childhood Cancer Survivor Study. Lancet Oncol. 2020;21:421–35. https://doi.org/10.1016/S1470-2045(19)30800-9. Epub 2020 Feb 14. PMID: 32066543; PMCID: PMC7392388.
Winther JF, Kenborg L, Byrne J, Hjorth L, Kaatsch P, Kremer LC, et al. Childhood cancer survivor cohorts in Europe. Acta Oncol. 2015;54:655–68. https://doi.org/10.3109/0284186X.2015.1008648. Epub 2015 Mar 27. PMID: 25813473.
Brinkman TM, Recklitis CJ, Michel G, Grootenhuis MA, Klosky JL. Psychological symptoms, social outcomes, socioeconomic attainment, and health behaviors among survivors of childhood cancer: current state of the literature. J Clin Oncol. 2018;36:2190–7. https://doi.org/10.1200/JCO.2017.76.5552. Epub 2018 Jun 6. PMID: 29874134; PMCID: PMC6053297.
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34:585–612. https://doi.org/10.1038/bjc.1976.220. PMID: 795448; PMCID: PMC2025229.
Cuzick J. Primary endpoints for randomised trials of cancer therapy. Lancet. 2008;371:2156–8. https://doi.org/10.1016/S0140-6736(08)60933-2. PMID: 18586160.
Cuzick J. The assessment of subgroups in clinical trials. Experientia Suppl. 1982;41:224–35. PMID: 6958512.
Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355:1064–9. https://doi.org/10.1016/S0140-6736(00)02039-0. PMID: 10744093.
Cuzick J. Forest plots and the interpretation of subgroups. Lancet. 2005;365:1308. https://doi.org/10.1016/S0140-6736(05)61026-4. PMID: 15823379.
Acknowledgements
I thank many colleagues for useful comments on earlier drafts, and especially Professor Stephen Duffy for those related to cancer screening.
Author information
Authors and Affiliations
Contributions
JC is the only author.
Corresponding author
Ethics declarations
Competing interests
I have developed an algorithm for predicting future breast cancer, which is freely available, but if used commercially requires a license from Cancer Research UK, and I receive a royalty from the income generated. No other conflicts to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cuzick, J. The importance of long-term follow up of participants in clinical trials. Br J Cancer 128, 432–438 (2023). https://doi.org/10.1038/s41416-022-02038-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-02038-4
This article is cited by
-
The comparison of functional status and health-related parameters in ovarian cancer survivors with healthy controls
Supportive Care in Cancer (2024)
-
Recent advances and future perspectives in the therapeutics of prostate cancer
Experimental Hematology & Oncology (2023)