Abstract
Background
This Phase 1b study (B2151002) evaluated the PI3K/mTOR inhibitor gedatolisib (PF-05212384) in combination with other anti-tumour agents in advanced solid tumours.
Methods
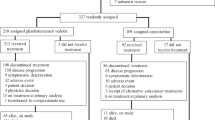
Patients with various malignancies were administered gedatolisib (90‒310 mg intravenously every week [QW]) plus docetaxel (arm A) or cisplatin (arm B) (each 75 mg/m2 intravenously Q3W) or dacomitinib (30 or 45 mg/day orally). The safety and tolerability of combination therapies were assessed during dose escalation; objective response (OR) and safety were assessed during dose expansion.
Results
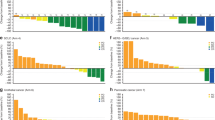
Of 110 patients enrolled, 107 received gedatolisib combination treatment. Seven of 70 (10.0%) evaluable patients had dose-limiting toxicities; the most common was grade 3 oral mucositis (n = 3). Based upon reprioritisation of the sponsor’s portfolio, dose expansion focused on arm B, gedatolisib (180 mg QW) plus cisplatin in patients (N = 22) with triple-negative breast cancer (TNBC). OR (95% CI) was achieved in four of ten patients in first-line (overall response rate 40.0% [12.2–73.8%]) and four of 12 in second/third-line (33.3% [9.9–65.1%]) settings. One patient in each TNBC arm (10%, first-line; 8.3%, second/third-line) achieved a complete response.
Conclusions
Gedatolisib combination therapy showed an acceptable tolerability profile, with clinical activity at the recommended Phase 2 dose in patients with TNBC.
Clinical trial
ClinicalTrial.gov: NCT01920061.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
On request, and subject to certain criteria, conditions and exceptions, Celcuity, Inc. will provide access to individual de-identified participant data from Celcuity sponsored interventional clinical studies conducted for medicines (1) for indications that have been approved or (2) where development has been terminated. A brief research proposal will be assessed and form the basis of a data-sharing agreement. Data requests may be submitted by using the form provided at https://www.celcuity.com/contact/. https://www.nature.com/documents/aj-research-data-policy-type-3.pdf.
Change history
25 January 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41416-023-02161-w
References
Gohr K, Hamacher A, Engelke LH, Kassack MU. Inhibition of PI3K/Akt/mTOR overcomes cisplatin resistance in the triple negative breast cancer cell line HCC38. BMC Cancer. 2017;17:711.
Khan KH, Yap TA, Yan L, Cunningham D. Targeting the PI3K-AKT-mTOR signaling network in cancer. Chin J Cancer. 2013;32:253–65.
Venkatesan AM, Dehnhardt CM, Delos Santos E, Chen Z, Dos Santos O, Ayral-Kaloustian S, et al. Bis(morpholino-1,3,5-triazine) derivatives: potent adenosine 5’-triphosphate competitive phosphatidylinositol-3-kinase/mammalian target of rapamycin inhibitors: discovery of compound 26 (PKI-587), a highly efficacious dual inhibitor. J Med Chem. 2010;53:2636–45.
Langdon SP, Kay C, Um IH, Dodds M, Muir M, Sellar G, et al. Evaluation of the dual mTOR/PI3K inhibitors gedatolisib (PF-05212384) and PF-04691502 against ovarian cancer xenograft models. Sci Rep. 2019;9:18742.
Shapiro GI, Bell-McGuinn KM, Molina JR, Bendell J, Spicer J, Kwak EL, et al. First-in-human study of PF-05212384 (PKI-587), a small-molecule, intravenous, dual inhibitor of PI3K and mTOR in patients with advanced cancer. Clin Cancer Res. 2015;21:1888–95.
Huang Y, Xue X, Li X, Jia B, Pan CX, Li Y, et al. Novel nanococktail of a dual PI3K/mTOR inhibitor and cabazitaxel for castration-resistant prostate cancer. Adv Ther. 2020;3:2000075.
Yan C, Yang J, Saleh N, Chen S-C, Ayers GD, Abramson VG, et al. Inhibition of the PI3K/mTOR pathway in breast cancer to enhance response to immune checkpoint inhibitors in breast cancer. Int J Mol Sci. 2021;22:5207.
Wainberg Z, Shapiro G, Curigliano G, Kristeleit R, Leong S, Alsina M, et al. Phase I study of the PI3K/mTOR inhibitor gedatolisib (PF-05212384) in combination with docetaxel, cisplatin, and dacomitinib. J Clin Oncol. 2016;34:2566.
Ji Y, Liu P, Li Y, Bekele BN. A modified toxicity probability interval method for dose-finding trials. Clin Trials. 2010;7:653–63.
Huang X, Biswas S, Oki Y, Issa JP, Berry DA. A parallel phase I/II clinical trial design for combination therapies. Biometrics. 2007;63:429–36.
Rodon J, Curigliano G, Delord JP, Harb W, Azaro A, Han Y, et al. A phase Ib, open-label, dose-finding study of alpelisib in combination with paclitaxel in patients with advanced solid tumors. Oncotarget. 2018;9:31709–18.
Sharma P, Abramson VG, O’Dea AP, Nye L, Mayer IA, Pathak HB, et al. Clinical and biomarker results from phase I/II study of PI3K inhibitor alpelisib plus nab-paclitaxel in HER2-negative metastatic breast cancer. Clin Cancer Res. 2021;27:3896–904.
Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24:628–37.
Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, Krag KJ, et al. TBCRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. 2015;33:1902–9.
Pascual J, Turner NC. Targeting the PI3-kinase pathway in triple-negative breast cancer. Ann Oncol. 2019;30:1051–60.
Choy L, Hagenbeek TJ, Solon M, French D, Finkle D, Shelton A, et al. Constitutive NOTCH3 signaling promotes the growth of basal breast cancers. Cancer Res. 2017;77:1439–52.
Hegde M, Joshi MB. Comprehensive analysis of regulation of DNA methyltransferase isoforms in human breast tumors. J Cancer Res Clin. 2021;147:937–71.
Li Y, Liu HT, Chen X, Wang YW, Tian YR, Ma RR, et al. Aberrant promoter hypermethylation inhibits RGMA expression and contributes to tumor progression in breast cancer. Oncogene. 2022;41:361–71.
Spangle JM, Roberts TM, Zhao JJ. The emerging role of PI3K/AKT-mediated epigenetic regulation in cancer. Biochim Biophys Acta Rev Cancer. 2017;1868:123–31.
Duffy MJ, Synnott NC, Crown J. Mutant p53 in breast cancer: potential as a therapeutic target and biomarker. Breast Cancer Res Tr. 2018;170:213–9.
Sporikova Z, Koudelakova V, Trojanec R, Hajduch M. Genetic markers in triple-negative breast cancer. Clin Breast Cancer. 2018;18:e841–e850.
Zhao ZM, Yost SE, Hutchinson KE, Li SM, Yuan YC, Noorbakhsh J, et al. CCNE1 amplification is associated with poor prognosis in patients with triple negative breast cancer. BMC Cancer. 2019;19:96.
Acknowledgements
The authors thank the patients, their families, and/or caregivers, investigators, and site personnel who have supported and contributed to this study. Eva Ciruelos at the University Hospital, Madrid, Spain, is acknowledged for her early contributions to this study. Medical writing support was provided by Sue Reinwald, PhD, of Engage Scientific Solutions (funded by Pfizer) and Michael G Baker, PhD, of Haselwood Biosciences (funded by Celcuity).
Funding
This study was sponsored by Pfizer.
Author information
Authors and Affiliations
Contributions
GC, GIS, EC, KK, KJP and HSR contributed to the conception and design of the study. All authors contributed to the acquisition, analysis, or interpretation of data, and to drafting or revising the submitted manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work. Dr. Curigliano (corresponding and co-first author) and Dr. Shapiro (co-first author) confirm they had full access to the data in the study and final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
GC: research funding: Merck; personal consultation fees: Pfizer, Roche, AstraZeneca, BMS, Daichii Sankyo, Seagen, Gilead, Novartis, Eli Lilly; Ellipsis, and Merck. GIS: research funding: Eli Lilly, Merck KGaA/EMD-Serono, Merck, and Sierra Oncology; Advisory boards: Pfizer, Eli Lilly, G1 Therapeutics, Roche, Merck KGaA/EMD-Serono, Sierra Oncology, Bicycle Therapeutics, Fusion Pharmaceuticals, Cybrexa Therapeutics, Astex, Almac, Ipsen, Bayer, Angiex, Daiichi Sankyo, Seattle Genetics, Boehringer Ingelheim, ImmunoMet, Asana, Artios, Atrin, Concarlo Holdings, Syros, Zentalis, CytomX Therapeutics and Blueprint Medicines; holds a patent titled “Dosage regimen for sapacitabine and seliciclib” also issued to Cyclacel Pharmaceuticals, and a pending patent titled “Compositions and Methods for Predicting Response and Resistance to CDK4/6 Inhibition” together with Liam Cornell. RSK has received grants from MSD and Clovis; Consultancy: Basilea, Pharmamar; Advisor: AstraZeneca, GSK, iTEOS, Eisai, and InCyte. ARAR: research funding: Pfizer. SL: research funding: institution funding as principal investigator on trials for Pfizer, BMS, Deciphera, and Karyopharma; employment: on faculty at University of Colorado at the time of this research, since June 2020 an employee of Merck. MA: consultancy: BMS, MSD, Lilly, and Servier. AG: author declared no conflict of interest. KAG: research funding: Pfizer, AstraZeneca, and BMS; advisory board member: Pfizer, Novartis, Eli Lilly, Roche, Merck, Mylan, AstraZeneca, Gilead, and Ayala. ES-R: research funding: Susan G. Komen, V Foundation and NIH; employment: principal or sub-investigator of Pfizer sponsor clinical trials, fees paid to the institution; consultancy fees: Eli Lilly, Merck, Macrogenics, and AstraZeneca. UNV: research support: Merck, BMS and Astellas Inc. Consulting and Honoraria: Merck, Exelixis, BMS, Pfizer, AAA, Alkermes, Bayer, and EMD-Serono. MM: Grants: Roche, AstraZeneca, GRAIL; grants and personal fees: GSK; personal fees and other: BiolineRx, BMS, Novartis; grants, personal fees and other: Immunocore; other: Pfizer, Regeneron; personal fees, non-financial support and other: Merck/MSD; personal fees and non-financial support: Replimune, personal fees: Kineta and Silicon Therapeutics. AJO: research funding: Pfizer, Merck, and BMS – compensation for ad board. HSR: research support: Pfizer, Merck, Novartis, Lilly, Roche, Daiichi, Seattle Genetics, Macrogenics, Sermonix, Boehringer Ingelheim, Polyphor, AstraZeneca, Ayala and Gilead; honoraria: Puma, Samsung and NAPO. KAK: an employee of and holds stock options in Pfizer. NP: research funding: Pfizer; an employee of and holds stock options in Pfizer. RP: Was an employee and held stock options in Pfizer at the time the work was done. KJP: an employee of and holds stock options in Pfizer. SCM: an employee of and holds stock options in Celcuity; holds stock in Alpine Immune Sciences. ZAW: research funding: Plexxikon, Novartis and BMS; advisory boards: AstraZeneca, Eli Lilly, Genentech, Merck KGaA/EMD-Serono, Ipsen, Bayer, Daiichi Sankyo, Seagen, Merck, BMS, Amgen, Five Prime and Macrogenics Molecular Templates.
Ethics approval and consent to participate
The protocol, its amendments, and informed consent documentation were reviewed and approved by the institutional review board(s) or independent ethics committee(s) at each site. Study procedures followed the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines and all local regulatory requirements. All patients provided informed consent before participating in any study-related procedure.
Consent to publish
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: As per Italian regulation, the two affiliations for Dr. Curigliano (Instituto Europeo di Oncologia, IRCCS and University of Milan, Milano, Italy) must be separated out for proper accreditation within their systems.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Curigliano, G., Shapiro, G.I., Kristeleit, R.S. et al. A Phase 1B open-label study of gedatolisib (PF-05212384) in combination with other anti-tumour agents for patients with advanced solid tumours and triple-negative breast cancer. Br J Cancer 128, 30–41 (2023). https://doi.org/10.1038/s41416-022-02025-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-02025-9
This article is cited by
-
PI3K/AKT/mTOR signaling pathway: an important driver and therapeutic target in triple-negative breast cancer
Breast Cancer (2024)
-
Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease
Signal Transduction and Targeted Therapy (2023)