Abstract
Background
Pancreatic cancer is among the most common malignant tumours, and effective therapeutic strategies are still lacking. While Corynoxine (Cory) can induce autophagy in neuronal cells, it remains unclear whether Cory has anti-tumour activities against pancreatic cancer.
Methods
Two pancreatic cancer cell lines, Patu-8988 and Panc-1, were used. Effects of Cory were evaluated by cell viability analysis, EdU staining, TUNEL assay, colony formation assay, and flow cytometry. Quantitative PCR and Western blot were performed to analyse mRNA and protein levels, respectively. In vivo anti-tumour efficacy of Cory was determined by a xenograft model.
Results
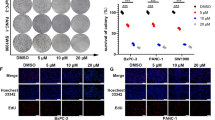
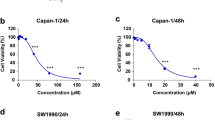
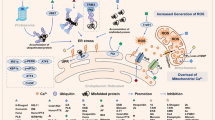
Cory treatment inhibited cell proliferation, induced endoplasmic reticulum (ER) stress, and triggered apoptosis in the pancreatic cancer cell lines. CHOP knockdown-mediated inhibition of ER stress alleviated the Cory-induced apoptosis but showed a limited effect on cell viability. Cory induced cell death partially via promoting reactive oxygen species (ROS) production and activating p38 signalling. Pretreatment with ROS scavenger N-acetylcysteine and p38 inhibitor SB203580 relieved the Cory-induced inhibition on cell growth. Cory remarkably blocked pancreatic tumour growth in vivo.
Conclusions
Cory exerts an anti-tumour effect on pancreatic cancer primarily via ROS-p38-mediated cytostatic effects. Cory may serve as a promising therapeutic agent for pancreatic cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data of this study are available within the article and in Supplementary Materials.
References
Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. 2021;71:209–49.
Chu L, Goggins M, Fishman E. Diagnosis and detection of pancreatic cancer. Cancer J. 2017;23:333–42.
Altman AM, Wirth K, Marmor S, Lou E, Denbo JW. Completion of adjuvant chemotherapy after upfront surgical resection for pancreatic cancer is uncommon yet associated with improved survival. Ann Surg Oncol. 2017;26:4108–16.
Mohammed S, Buren GV, Fisher WE. Pancreatic cancer: advances in treatment. World J Gastroenterol. 2014;10:2351–62.
Yan D, Ma Z, Liu C, Wang C, Deng Y, Liu W, et al. Corynoxine B ameliorates HMGB1-dependent autophagy dysfunction during manganese exposure in SH-SY5Y human neuroblastoma cells. Food Chem Toxicol. 2018;124:336–48.
Chen LL, Song JX, Lu JH, Yuan ZW, Liu LF, Durairajan S, et al. Corynoxine, a natural autophagy enhancer, promotes the clearance of alpha-synuclein via Akt/mTOR pathway. J Neuroimmune Pharm. 2014;9:380–7.
Kim TJ, Lee JH, Lee JJ, Yu JY, Hwang BY, Ye SK, et al. Corynoxeine isolated from the hook of Uncaria rhynchophylla inhibits rat aortic vascular smooth muscle cell proliferation through the blocking of extracellular signal regulated kinase 1/2 phosphorylation. Biol Pharm Bull. 2008;31:2073–82.
Zhou Y, Tang M, Liu S. Reversal effect of isorhynchophylline on lung adenocarcinoma drug-resistant cell line A549/DDP. Chin J N Drugs. 2009;18:1338–42.
Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–22.
Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl JA, Majsterek I. The role of the PERK/EIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr Mol Med. 2016;16:533–44.
Li HY, Zhang J, Sun LL, Li BH, Gao HL, Xie T, et al. Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells: an in vitro and in vivo study. Cell Death Dis. 2015;6:1604–5.
Wang H, Zhang T, Sun W, Wang Z, Zuo D, Zhou Z, et al. Erianin induces G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2016;7:2247–56.
Chen YQ, Mcmillan-Ward E, Kon GJM, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171–9.
Poillet-Perez L, Despouy G, Delage-Mourroux R, Boyer-Guittaut M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 2015;4:184–92.
Lin CL, Lee CH, Chen CM, Cheng CW, Chen PN, Ying TH, et al. Protodioscin induces apoptosis through ROS-mediated endoplasmic reticulum stress via the JNK/p38 activation pathways in human cervical cancer cells. Cell Physiol Biochem. 2018;13:322–34.
Heo JR, Kim SM, Hwang KA, Kang JH, Choi KC. Resveratrol induced reactive oxygen species and endoplasmic reticulum stressmediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. Int J Mol Med. 2018;42:1427–35.
Wang YY, Lee KT, Lim MC, Choi JH. TRPV1 antagonist DWP05195 induces ER stress-dependent apoptosis through the ROS-p38-CHOP pathway in human ovarian cancer cells. Cancers. 2020;12:1702–10.
He JH, Liu RP, Peng YM, Guo Q, Zhu LB, Lian YZ, et al. Differential and paradoxical roles of new-generation antidepressants in primary astrocytic inflammation. J Neuroinflamm. 2021;18:47–55.
Shao ZQ, Zhang X, Fan HH, Wan GXS, Wu HM, Zhang L, et al. Selenoprotein T promotes proliferation and G1-to-S transition in SK-N-SH cells: implications in Parkinson’s disease. J Nutr. 2019;149:2110–9.
Zhang HQ, Wang JY, Li ZF, Cui L, Huang SS, Zhu LB, et al. DNA methyltransferase 1 is dysregulated in parkinson’s disease via mediation of miR-17. Mol Neurobiol. 2021;58:2620–33.
Yokouchi M, Hiramatsu N, Hayakawa K, Okamura M, Du S, Kasai A, et al. Involvement of selective reactive oxygen species upstream of proapoptotic branches of unfolded protein response. J Biol Chem. 2008;283:4252–60.
Kim B, Kim HS, Jung EJ, Lee JY, Tsang BK, Lim JM, et al. Curcumin induces ER stress-mediated apoptosis through selective generation of reactive oxygen species in cervical cancer cells. Mol Carcinog. 2016;55:918–28.
Zhong H, Song R, Pang Q, Liu Y, Zhuang J, Chen Y. Propofol inhibits parthanatos via ROS-ER-calcium-mitochondria signal pathway in vivo and vitro. Cell Death Dis. 2018;9:1804–20.
Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;20:253–62.
Lee YS, Lee DH, Choudry HA, Bartlett DL, Lee YJ. Ferroptosis-induced endoplasmic reticulum stress: cross-talk between ferroptosis and apoptosis. Mol Cancer Res. 2018;16:1073–6.
Lin SS, Huang HP, Yang JS, Wu JY, Hsai TC, Lin CC, et al. DNA damage and endoplasmic reticulum stress mediated curcumin-induced cell cycle arrest and apoptosis in human lung carcinoma A-549 cells through the activation caspases cascade- and mitochondrial-dependent pathway. Cancer Lett. 2008;272:77–90.
Matsumoto M, Minami M, Takeda K, Sakao Y, Akira S. Ectopic expression of CHOP (GADD153) induces apoptosis in M1 myeloblastic leukemia cells. FEBS Lett. 1996;395:23–40.
Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–55.
Wang Y, Xiao J, Zhou H, Yang S, Wu X, Jiang C, et al. A novel monocarbonyl analogue of curcumin, (1E,4E)-1,5-bis(2,3-dimethoxyphenyl) penta-1,4-dien-3-one, induced cancer cell H460 apoptosis via activation of endoplasmic reticulum stress signaling pathway. J Med Chem. 2011;54:3768–78.
Deniaud A, Dein O, Maillier E, Poncet D, Kroemer G, Lemaire C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;10:1732–44.
Kirtonia A, Sethi G, Garg M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell Mol Life Sci. 2020;77:4459–83.
Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–93.
Chen W, Li P, Liu Y, Yang Y, Ye XT, Zhang F, et al. Isoalantolactone induces apoptosis through ROS-mediated ER stress and inhibition of STAT3 in prostate cancer cells. J Exp Clin Cancer Res. 2018;37:309–18.
Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors. 2010;17:604–15.
Park GB, Kim YS, Lee HK, Song H, Kim S, Cho DH, et al. Reactive oxygen species and p38 MAPK regulate Bax translocation and calcium redistribution in salubrinal-induced apoptosis of EBV-transformed B cells. Cancer Lett. 2011;313:235–48.
Liu YQ, Liu YF, Xiao YD, Wang YB, Zhang X. Hydrogen-rich saline attenuates skin ischemia/reperfusion induced apoptosis via regulating Bax/Bcl-2 ratio and ASK-1/JNK pathway. J Plast Reconstr Aesth Surg. 2015;68:147–56.
Markou T, Dowling AA, Kelly T, Lazou A. Regulation of Bcl-2 phosphorylation in response to oxidative stress in cardiac myocytes. Free Radic Res. 2009;43:809–16.
Qiang W, Wang H, Yue J, Hao P, Hui D. Luteolin induces apoptosis by ROS/ER stress and mitochondrial dysfunction in gliomablastoma. Cancer Chemother Pharm. 2017;79:1031–41.
Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90.
Haagenson KK, Wu GS. Mitogen activated protein kinase phosphatases and cancer. Cancer Biol Ther. 2010;9:337–40.
Feaver RE, Hastings NE, Pryor A, Blackman BR. GRP78 upregulation by atheroprone shear stress via p38-, α2β1-dependent mechanism in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1534–41.
Ranganathan AC, Zhang L, Adam AP, Aguirre-Ghiso JA. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase–like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 2006;66:1702–11.
Jiang Q, Li F, Shi K, Wu P, An J, Yang Y, et al. Involvement of p38 in signal switching from autophagy to apoptosis via the PERK/eIF2α/ATF4 axis in selenite-treated NB4 cells. Cell Death Dis. 2014;5:1270–83.
Do MT, Na M, Kim HG, Khanal T, Choi JH, Jin SW, et al. Ilimaquinone induces death receptor expression and sensitizes human colon cancer cells to TRAIL-induced apoptosis through activation of ROS-ERK/p38 MAPK–CHOP signaling pathways. Food Chem Toxicol. 2014;71:51–9.
Ying L, Liu Z, Guo X, Jian S, Chen Z, Li L. Aristolochic acid I-induced DNA damage and cell cycle arrest in renal tubular epithelial cells in vitro. Arch Toxicol. 2006;80:524–32.
Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–43.
Wang K, Ma JY, Li MY, Qin YS, Bao XC, Wang CC, et al. Mechanisms of Cd and Cu induced toxicity in human gastric epithelial cells: oxidative stress, cell cycle arrest and apoptosis. Sci Total Environ. 2021;14:1439–50.
Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–4.
Shom G, Decristo MJ, Mcallister SS, Zhao JJ. CDK4/6 inhibition in cancer: beyond cell cycle arrest. Trends Cell Biol. 2018;28:911–25.
Kawasumi M, Bradner JE, Tolliday N, Thibodeau R, Sloan H, Brummond KM, et al. Identification of ATR-Chk1 pathway inhibitors that selectively target p53-deficient cells without directly suppressing ATR catalytic activity. Cancer Res. 2014;74:75–84.
Palla VV, Karaolanis G, Katafigiotis I, Anastasiou I, Patapis P, Dimitroulis D, et al. gamma-H2AX: can it be established as a classical cancer prognostic factor? Tumour Biol. 2017;39:2831–44.
Valdiglesias V, Giunta S, Fenech M, Neri M, Bonassi S. γH2AX as a marker of DNA double strand breaks and genomic instability in human population studies. Mutat Res. 2013;753:24–40.
Chen X, Kang R, Kroemer G, Tang D. Targeting ferroptosis in pancreatic cancer: a double-edged sword. Trends Cancer. 2021;7:636–49.
Latunde-Dada GO. Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj. 2017;1861:1893–900.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 82102983), Wenzhou Municipal Science and Technology Bureau (grant number Y20210234), and the Fundamental Research Funds for Wenzhou Medical University (grant number KYYW202019).
Author information
Authors and Affiliations
Contributions
JD, ZX, and XY designed the project; CW, QR, and XY performed the molecular biology and cell biology experiments and analysed data. ZL and XZ performed in vivo experiments. JD, ZX, and CW wrote the manuscript. PDPA contributed to the manuscript editing. All authors contributed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal procedures were performed according to the Institutional laboratory animal research guidelines and were approved by Wenzhou Medical University Animal Policy and Welfare Committee. This study conformed to the principles outlined in the Declaration of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wen, C., Ruan, Q., Li, Z. et al. Corynoxine suppresses pancreatic cancer growth primarily via ROS-p38 mediated cytostatic effects. Br J Cancer 127, 2108–2117 (2022). https://doi.org/10.1038/s41416-022-02002-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-02002-2
This article is cited by
-
Suppressing MTERF3 inhibits proliferation of human hepatocellular carcinoma via ROS-mediated p38 MAPK activation
Communications Biology (2024)