Abstract
Background
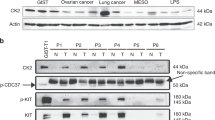
Advanced gastrointestinal stromal tumour (GIST) is characterised by genomic perturbations of key cell cycle regulators. Oncogenic activation of CDK4/6 results in RB1 inactivation and cell cycle progression. Given that single-agent CDK4/6 inhibitor therapy failed to show clinical activity in advanced GIST, we evaluated strategies for maximising response to therapeutic CDK4/6 inhibition.
Methods
Targeted next-generation sequencing and multiplexed protein imaging were used to detect cell cycle regulator aberrations in GIST clinical samples. The impact of inhibitors of CDK2, CDK4 and CDK2/4/6 was determined through cell proliferation and protein detection assays. CDK-inhibitor resistance mechanisms were characterised in GIST cell lines after long-term exposure.
Results
We identify recurrent genomic aberrations in cell cycle regulators causing co-activation of the CDK2 and CDK4/6 pathways in clinical GIST samples. Therapeutic co-targeting of CDK2 and CDK4/6 is synergistic in GIST cell lines with intact RB1, through inhibition of RB1 hyperphosphorylation and cell proliferation. Moreover, RB1 inactivation and a novel oncogenic cyclin D1 resulting from an intragenic rearrangement (CCND1::chr11.g:70025223) are mechanisms of acquired CDK-inhibitor resistance in GIST.
Conclusions
These studies establish the biological rationale for CDK2 and CDK4/6 co-inhibition as a therapeutic strategy in patients with advanced GIST, including metastatic GIST progressing on tyrosine kinase inhibitors.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data generated in this study are publicly available in GenBank (NCBI) at accession no. 2511709, Gene Expression Omnibus (GEO) at accession number GSE206257, and within the article and its Supplementary data files. As per genomic data sharing guidelines, sequence and assay data are deposited in a publicly available database sponsored by the American Association for Cancer Research (AACR) Project GENIE. Variant calls and a limited clinical dataset from patients are sent to the Synapse platform, developed by Sage Bionetworks, where the data are harmonised and protected health information (PHI) removed in a secure Health Insurance Portability and Accountability Act (HIPAA)-the compliant environment that provides data governance.
References
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80.
Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10.
Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–6.
Heinrich MC, Rankin C, Blanke CD, Demetri GD, Borden EC, Ryan CW, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results: analysis of phase 3 SWOG intergroup trial S0033. JAMA Oncol. 2017;3:944–52.
Liegl B, Kepten I, Le C, Zhu M, Demetri GD, Heinrich MC, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol. 2008;216:64–74.
Wardelmann E, Merkelbach-Bruse S, Pauls K, Thomas N, Schildhaus HU, Heinicke T, et al. Polyclonal evolution of multiple secondary KIT mutations in gastrointestinal stromal tumors under treatment with imatinib mesylate. Clin Cancer Res. 2006;12:1743–9.
Schaefer IM, Wang Y, Liang CW, Bahri N, Quattrone A, Doyle L, et al. MAX inactivation is an early event in GIST development that regulates p16 and cell proliferation. Nat Commun. 2017;8:14674.
Pang Y, Xie F, Cao H, Wang C, Zhu M, Liu X, et al. Mutational inactivation of mTORC1 repressor gene DEPDC5 in human gastrointestinal stromal tumors. Proc Natl Acad Sci USA. 2019;116:22746–53.
Wozniak A, Sciot R, Guillou L, Pauwels P, Wasag B, Stul M, et al. Array CGH analysis in primary gastrointestinal stromal tumors: cytogenetic profile correlates with anatomic site and tumor aggressiveness, irrespective of mutational status. Genes Chromosomes Cancer. 2007;46:261–76.
Heinrich MC, Patterson J, Beadling C, Wang Y, Debiec-Rychter M, Dewaele B, et al. Genomic aberrations in cell cycle genes predict progression of KIT-mutant gastrointestinal stromal tumors (GISTs). Clin Sarcoma Res. 2019;9:3.
Wang Y, Marino-Enriquez A, Bennett RR, Zhu M, Shen Y, Eilers G, et al. Dystrophin is a tumor suppressor in human cancers with myogenic programs. Nat Genet. 2014;46:601–6.
Narasimha AM, Kaulich M, Shapiro GS, Choi YJ, Sicinski P, Dowdy SF. Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. eLife. 2014;3:e02872.
Haller F, Gunawan B, von Heydebreck A, Schwager S, Schulten HJ, Wolf-Salgo J, et al. Prognostic role of E2F1 and members of the CDKN2A network in gastrointestinal stromal tumors. Clin Cancer Res. 2005;11:6589–97.
Perrone F, Tamborini E, Dagrada GP, Colombo F, Bonadiman L, Albertini V, et al. 9p21 locus analysis in high-risk gastrointestinal stromal tumors characterized for c-kit and platelet-derived growth factor receptor alpha gene alterations. Cancer. 2005;104:159–69.
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35.
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–46.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–48.
Toulmonde M, Blay JY, Bouche O, Mir O, Penel N, Isambert N, et al. Activity and safety of palbociclib in patients with advanced gastrointestinal stromal tumors refractory to imatinib and sunitinib: a biomarker-driven phase II study. Clin Cancer Res. 2019;25:4611–5.
Pandey K, Park N, Park KS, Hur J, Cho YB, Kang M, et al. Combined CDK2 and CDK4/6 inhibition overcomes palbociclib resistance in breast cancer by enhancing senescence. Cancers. 2020;12:3566.
Saka SK, Wang Y, Kishi JY, Zhu A, Zeng Y, Xie W, et al. Immuno-SABER enables highly multiplexed and amplified protein imaging in tissues. Nat Biotechnol. 2019;37:1080–90.
Kishi JY, Schaus TE, Gopalkrishnan N, Xuan F, Yin P. Programmable autonomous synthesis of single-stranded DNA. Nat Chem. 2018;10:155–64.
Kishi JY, Lapan SW, Beliveau BJ, West ER, Zhu A, Sasaki HM, et al. SABER amplifies FISH: enhanced multiplexed imaging of RNA and DNA in cells and tissues. Nat Methods. 2019;16:533–44.
Muhlich J, Chen Y-A, Russell D, Sorger PK. Stitching and registering highly multiplexed whole slide images of tissues and tumors using ASHLAR. Bioinformatics. 2022:btac544. https://doi.org/10.1093/bioinformatics/btac544. Online ahead of print.
Burel JM, Besson S, Blackburn C, Carroll M, Ferguson RK, Flynn H, et al. Publishing and sharing multi-dimensional image data with OMERO. Mamm Genome. 2015;26:441–7.
Schaefer IM, Lundberg MZ, Demicco EG, Przybyl J, Matusiak M, Chibon F, et al. Relationships between highly recurrent tumor suppressor alterations in 489 leiomyosarcomas. Cancer. 2021;127:2666–73.
Rubin BP, Singer S, Tsao C, Duensing A, Lux ML, Ruiz R, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118–21.
Wagle N, Berger MF, Davis MJ, Blumenstiel B, Defelice M, Pochanard P, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2:82–93.
Garcia EP, Minkovsky A, Jia Y, Ducar MD, Shivdasani P, Gong X, et al. Validation of OncoPanel: a targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med. 2017;141:751–8.
Paulo, JA. Sample preparation for proteomic analysis using a GeLC-MS/MS strategy. J Biol Methods. 2016;3:e45.
Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1:2856–60.
Zheng S, Wang W, Aldahdooh J, Malyutina A, Shadbahr T, Tanoli Z, et al. SynergyFinder plus: toward better interpretation and annotation of drug combination screening datasets. Genomics Proteomics Bioinformat. 2022. https://doi.org/10.1016/j.gpb.2022.01.004.
Malyutina A, Majumder MM, Wang W, Pessia A, Heckman CA, Tang J. Drug combination sensitivity scoring facilitates the discovery of synergistic and efficacious drug combinations in cancer. PLoS Comput Biol. 2019;15:e1006752.
Ou WB, Ni N, Zuo R, Zhuang W, Zhu M, Kyriazoglou A, et al. Cyclin D1 is a mediator of gastrointestinal stromal tumor KIT-independence. Oncogene. 2019;38:6615–29.
Freeman-Cook KD, Hoffman RL, Behenna DC, Boras B, Carelli J, Diehl W, et al. Discovery of PF-06873600, a CDK2/4/6 inhibitor for the treatment of cancer. J Med Chem. 2021;64:9056–77.
Asghar US, Barr AR, Cutts R, Beaney M, Babina I, Sampath D, et al. Single-cell dynamics determines response to CDK4/6 inhibition in triple-negative breast cancer. Clin Cancer Res. 2017;23:5561–72.
Chandarlapaty S, Razavi P. Cyclin E mRNA: assessing cyclin-dependent kinase (CDK) activation state to elucidate breast cancer resistance to CDK4/6 inhibitors. J Clin Oncol. 2019;37:1148–50.
Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76:2301–13.
Min A, Kim JE, Kim YJ, Lim JM, Kim S, Kim JW, et al. Cyclin E overexpression confers resistance to the CDK4/6 specific inhibitor palbociclib in gastric cancer cells. Cancer Lett. 2018;430:123–32.
Guarducci C, Bonechi M, Benelli M, Biagioni C, Boccalini G, Romagnoli D, et al. Cyclin E1 and Rb modulation as common events at time of resistance to palbociclib in hormone receptor-positive breast cancer. NPJ Breast Cancer. 2018;4:38.
Yang C, Li Z, Bhatt T, Dickler M, Giri D, Scaltriti M, et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene. 2017;36:2255–64.
Condorelli R, Spring L, O’Shaughnessy J, Lacroix L, Bailleux C, Scott V, et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2018;29:640–5.
O’Leary B, Cutts RJ, Liu Y, Hrebien S, Huang X, Fenwick K, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 2018;8:1390–403.
Gong X, Du J, Parsons SH, Merzoug FF, Webster Y, Iversen PW, et al. Aurora A kinase inhibition is synthetic lethal with loss of the RB1 tumor suppressor gene. Cancer Discov. 2019;9:248–63.
Lyu J, Yang EJ, Zhang B, Wu C, Pardeshi L, Shi C, et al. Synthetic lethality of RB1 and aurora A is driven by stathmin-mediated disruption of microtubule dynamics. Nat Commun. 2020;11:5105.
Lu Z, Bauzon F, Fu H, Cui J, Zhao H, Nakayama K, et al. Skp2 suppresses apoptosis in Rb1-deficient tumours by limiting E2F1 activity. Nat Commun. 2014;5:3463.
Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24.
Montaudon E, Nikitorowicz-Buniak J, Sourd L, Morisset L, El Botty R, Huguet L, et al. PLK1 inhibition exhibits strong anti-tumoral activity in CCND1-driven breast cancer metastases with acquired palbociclib resistance. Nat Commun. 2020;11:4053.
Simoneschi D, Rona G, Zhou N, Jeong YT, Jiang S, Milletti G, et al. CRL4(AMBRA1) is a master regulator of D-type cyclins. Nature. 2021;592:789–93.
Freeman-Cook K, Hoffman RL, Miller N, Almaden J, Chionis J, Zhang Q, et al. Expanding control of the tumor cell cycle with a CDK2/4/6 inhibitor. Cancer Cell. 2021;39:1404–21.e1411.
Kumarasamy V, Vail P, Nambiar R, Witkiewicz AK, Knudsen ES. Functional determinants of cell cycle plasticity and sensitivity to CDK4/6 inhibition. Cancer Res. 2021;81:1347–60.
Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77.
Watt AC, Goel S. Cellular mechanisms underlying response and resistance to CDK4/6 inhibitors in the treatment of hormone receptor-positive breast cancer. Breast Cancer Res. 2022;24:17.
Choi YJ, Li X, Hydbring P, Sanda T, Stefano J, Christie AL, et al. The requirement for cyclin D function in tumor maintenance. Cancer Cell. 2012;22:438–51.
Flavahan WA, Drier Y, Johnstone SE, Hemming ML, Tarjan DR, Hegazi E, et al. Altered chromosomal topology drives oncogenic programs in SDH-deficient GISTs. Nature. 2019;575:229–33.
Urbini M, Astolfi A, Indio V, Nannini M, Schipani A, Bacalini MG, et al. Gene duplication, rather than epigenetic changes, drives FGF4 overexpression in KIT/PDGFRA/SDH/RAS-P WT GIST. Sci Rep. 2020;10:19829.
Kong T, Xue Y, Cencic R, Zhu X, Monast A, Fu Z, et al. eIF4A inhibitors suppress cell-cycle feedback response and acquired resistance to CDK4/6 inhibition in cancer. Mol Cancer Ther. 2019;18:2158–70.
Acknowledgements
The authors thank Dr. Peter Sicinski, Dana-Farber Cancer Institute, for facilitating the cell cycle phase analyses and for critical review of the manuscript and Dr. Geoffrey I. Shapiro, Dana-Farber Cancer Institute, for critical review of the manuscript.
Funding
This work is supported by the NCI K08CA241085 grant (IMS), SARC (Sarcoma Alliance for Research through Collaboration) Young Investigator Award (IMS), the Wyss Institute Validation Project Program and the Office of Naval Research grant N00014-18-1-2549 (PY), the NIGMS R01GM132129 grant (JAP), the NIH UH3CA25513303 and 1DP1GM133052 grants (SKS, IG, MPS, and PY), Pfizer and Lilly through the Alliance for Clinical Trials in Oncology (MMB) and the European Molecular Biology Laboratory (EMBL) core funding (SKS).
Author information
Authors and Affiliations
Contributions
IMS and JAF designed the study. IMS, MLH, MZL, MPS, IG, JAP, AME, WBO and SKS performed the experiments and data analysis with assistance from SPG, NL and JLH. IMS and JAF supervised the study. JAP, SPG, SG, JAM, MMB, ETA, AME, JLH, CPR, GDD, SKS and JAF provided resources. IMS drafted the manuscript. MLH, MZL, JAP, SG, MMB, AME, WBO, GDD, SKS and JAF edited the manuscript. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
SKS and PY are inventors for a US patent application for the multiplexed imaging technology used in this work. PY is a co-founder of Ultivue, Inc. and NuProbe Global. SG serves as consultant/advisory board member to Deciphera Pharmaceuticals, Blueprint Medicines, Daiichi-Sankyo, Kayothera, Immunicum, Eli Lilly, Bayer, Ayala; reports research funding to the institution by Deciphera Pharmaceuticals, Blueprint Medicines, Daiichi-Sankyo, Theseus Pharmaceuticals, Merck, Eisai, Springworks, Pfizer and Bayer; holds equity at Abbott Laboratories; and receives royalties from Wolters Kluwer/UpToDate. MMB serves on the Board of Directors of Natera, Inc., and Leap Therapeutics. JLH serves as a consultant to Aadi Biosciences and TRACON Pharmaceuticals. GDD serves as a Board of Directors member with minor equity holding in Blueprint Medicines; serves as co-founder with minor equity holding in IDRX; serves as consultant/SAB member with minor equity holding in G1 Therapeutics, Caris Life Sciences, Erasca Pharmaceuticals, RELAY Therapeutics, Bessor Pharmaceuticals, CellCarta, IKENA Oncology, Kojin Therapeutics, Acrivon Therapeutics; serves as a scientific consultant with sponsored research to Dana-Farber to Bayer, Pfizer, Novartis, Roche/Genentech, Janssen, PharmaMar, Daiichi-Sankyo, AdaptImmune; serves as a scientific consultant to GlaxoSmithKline, EMD-Serono, MEDSCAPE, Mirati, WCG/Arsenal Capital, RAIN Therapeutics; and receives Novartis royalty to Dana-Farber for use patent of imatinib in GIST. The remaining authors declare no competing interests.
Ethics approval and consent to participate
Patient sample collection and analysis were conducted following protocols approved by the Dana-Farber/Brigham and Women’s Hospital Institutional Review Board. Written informed consent was obtained from patients for use of samples. The study was performed in accordance with the Declaration of Helsinki.
Consent to publish
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schaefer, IM., Hemming, M.L., Lundberg, M.Z. et al. Concurrent inhibition of CDK2 adds to the anti-tumour activity of CDK4/6 inhibition in GIST. Br J Cancer 127, 2072–2085 (2022). https://doi.org/10.1038/s41416-022-01990-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01990-5
This article is cited by
-
PROTAC-mediated CDK degradation differentially impacts cancer cell cycles due to heterogeneity in kinase dependencies
British Journal of Cancer (2023)