Abstract
Background
The aim of this study was to gain an increased understanding of the aetiology of breast cancer, by investigating possible associations between serum lipoprotein subfractions and metabolites and the long-term risk of developing the disease.
Methods
From a cohort of 65,200 participants within the Trøndelag Health Study (HUNT study), we identified all women who developed breast cancer within a 22-year follow-up period. Using nuclear magnetic resonance (NMR) spectroscopy, 28 metabolites and 89 lipoprotein subfractions were quantified from prediagnostic serum samples of future breast cancer patients and matching controls (n = 1199 case–control pairs).
Results
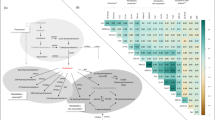
Among premenopausal women (554 cases) 14 lipoprotein subfractions were associated with long-term breast cancer risk. In specific, different subfractions of VLDL particles (in particular VLDL-2, VLDL-3 and VLDL-4) were inversely associated with breast cancer. In addition, inverse associations were detected for total serum triglyceride levels and HDL-4 triglycerides. No significant association was found in postmenopausal women.
Conclusions
We identified several associations between lipoprotein subfractions and long-term risk of breast cancer in premenopausal women. Inverse associations between several VLDL subfractions and breast cancer risk were found, revealing an altered metabolism in the endogenous lipid pathway many years prior to a breast cancer diagnosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The Trøndelag Health Study (HUNT) has invited persons aged 13–100 years to four surveys between 1984 and 2019. Comprehensive data from more than 140,000 persons having participated at least once and biological material from 78,000 persons are collected. The data are stored in HUNT databank and biological material in HUNT biobank. HUNT Research Centre has permission from the Norwegian Data Inspectorate to store and handle these data. The key identification in the database is the personal identification number given to all Norwegians at birth or immigration, whilst de-identified data are sent to researchers upon approval of a research protocol by the Regional Ethical Committee and HUNT Research Centre. To protect participants’ privacy, HUNT Research Centre aims to limit the storage of data outside HUNT databank, and cannot deposit data in open repositories. HUNT databank has precise information on all data exported to different projects and are able to reproduce these on request. There are no restrictions regarding data export given approval of applications to HUNT Research Centre. For more information, see http://www.ntnu.edu/hunt/data.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Cancer Registry of Norway. Cancer in Norway 2018- Cancer incidence, mortality, survival and prevalence in Norway. Oslo: Cancer Registry of Norway, 2019. https://www.kreftregisteret.no/globalassets/cancer-in-norway/2018/cin_report.pdf.
van Roekel EH, Trijsburg L, Assi N, Carayol M, Achaintre D, MurphyN, et al. Circulating metabolites associated with alcohol intake in the European Prospective Investigation into Cancer and Nutrition Cohort. Nutrients. 2018;10:654.
Carayol M, Leitzmann MF, Ferrari P, Zamora-Ros R, Achaintre D, Stepien M, et al. Blood metabolic signatures of body mass index: a targeted metabolomics study in the EPIC cohort. J Proteome Res. 2017;16:3137–46.
Bathen TF, Geurts B, Sitter B, Fjosne HE, Lundgren S, Buydens LM, et al. Feasibility of MR metabolomics for immediate analysis of resection margins during breast cancer surgery. PLoS ONE. 2013;8:e61578.
Gu H, Pan Z, Xi B, Asiago V, Musselman B, Raftery D. Principal component directed partial least squares analysis for combining nuclear magnetic resonance and mass spectrometry data in metabolomics: application to the detection of breast cancer. Anal Chim Acta. 2011;686:57–63.
Slupsky CM, Steed H, Wells TH, Dabbs K, Schepansky A, Capstick V, et al. Urine metabolite analysis offers potential early diagnosis of ovarian and breast cancers. Clin Cancer Res. 2010;16:5835–41.
Furberg AS, Veierod MB, Wilsgaard T, Bernstein L, Thune I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst. 2004;96:1152–60.
Bro R, Kamstrup-Nielsen MH, Engelsen SB, Savorani F, Rasmussen MA, Hansen L, et al. Forecasting individual breast cancer risk using plasma metabolomics and biocontours. Metabolomics. 2015;11:1376–80.
His M, Viallon V, Dossus L, Gicquiau A, Achaintre D, Scalbert A, et al. Prospective analysis of circulating metabolites and breast cancer in EPIC. BMC Med. 2019;17:178.
Lecuyer L, Victor Bala A, Deschasaux M, Bouchemal N, Nawfal Triba M, Vasson MP, et al. NMR metabolomic signatures reveal predictive plasma metabolites associated with long-term risk of developing breast cancer. Int J Epidemiol. 2018;47:484–94.
Kuhn T, Floegel A, Sookthai D, Johnson T, Rolle-Kampczyk U, Otto W, et al. Higher plasma levels of lysophosphatidylcholine 18:0 are related to a lower risk of common cancers in a prospective metabolomics study. BMC Med. 2016;14:13.
Moore SC, Playdon MC, Sampson JN, Hoover RN, Trabert B, Matthews CE, et al. A metabolomics analysis of body mass index and postmenopausal breast cancer risk. J Natl Cancer Inst. 2018;110:588–97.
Playdon MC, Ziegler RG, Sampson JN, Stolzenberg-Solomon R, Thompson HJ, Irwin ML, et al. Nutritional metabolomics and breast cancer risk in a prospective study. Am J Clin Nutr. 2017;106:637–49.
Zeleznik OA, Balasubramanian R, Zhao Y, Frueh L Jeanfavre S, Avila-Pacheco J, et al. Circulating amino acids and amino acid-related metabolites and risk of breast cancer among predominantly premenopausal women. npj Breast Cancer. 2021;7:1–10.
Lecuyer L, Dalle C, Lyan B, Demidem A, Rossary A, Vasson MP, et al. Plasma metabolomic signatures associated with long-term breast cancer risk in the SU.VI.MAX prospective cohort. Cancer Epidemiol Biomark Prev. 2019;28:1300–7.
Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech. 2013;6:1353–63.
Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–23.
Feingold KR, Grunfeld, C. Introduction to lipids and lipoproteins. in Endotext [Internet]. Feingold, KR, et al. South Dartmouth, MA, USA: MDText.com, Inc.; 2020.
Krokstad S, Langhammer A, Hveem K, Holmen TL, Midthjell K, Stene TR, et al. Cohort profile: the HUNT study, Norway. Int J Epidemiol. 2013;42:968–77.
Dona AC, Jimenez B, Schafer H, Humpfer E, Spraul M, Lewis MR, et al. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal Chem. 2014;86:9887–94.
Tomasi G, Savorani F, Engelsen SB. icoshift: an effective tool for the alignment of chromatographic data. J Chromatogr A. 2011;1218:7832–40.
Cloarec O, Dumas ME, Craig A, Barton RH, Trygg J, Hudson J, et al. Statistical total correlation spectroscopy: an exploratory approach for latent biomarker identification from metabolic 1H NMR data sets. Anal Chem. 2005;77:1282–9.
Jimenez B, Holmes E, Heude C, Tolson RF, Harvey N, Lodge SL, et al. Quantitative lipoprotein subclass and low molecular weight metabolite analysis in human serum and plasma by (1)H NMR spectroscopy in a multilaboratory trial. Anal Chem. 2018;90:11962–71.
Bjelland EK, Hofvind S, Byberg L, Eskild A. The relation of age at menarche with age at natural menopause: a population study of 336 788 women in Norway. Hum Reprod. 2018;33:1149–57.
Clavel-Chapelon F, Group EN. Cumulative number of menstrual cycles and breast cancer risk: results from the E3N cohort study of French women. Cancer Causes Control: CCC. 2002;13:831–8.
Collaborative Group on Hormonal Factors in Breast, C. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047–59.
Collaborative Group on Hormonal Factors in Breast, C. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394:1159–68.
Lacey JV Jr, Kreimer AR, Buys SS, Marcus PM, Chang SC, Leitzmann MF, et al. Breast cancer epidemiology according to recognized breast cancer risk factors in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial Cohort. BMC Cancer. 2009;9:84.
Morrisett JD, Jackson RL, Gotto AM Jr. Lipoproteins: structure and function. Annu Rev Biochem. 1975;44:183–207.
Jobard E, Dossus L, Baglietto L, Fornili M, Lecuyer L, Mancini FR, et al. Investigation of circulating metabolites associated with breast cancer risk by untargeted metabolomics: a case-control study nested within the French E3N cohort. Br J Cancer. 2021;124:1734–43.
Bendinelli B, Vignoli A, Palli D, Assedi M, Ambrogetti D, Luchinat C, et al. Prediagnostic circulating metabolites in female breast cancer cases with low and high mammographic breast density. Sci Rep. 2021;11:13025.
Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, et al. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14:1009–19.
Key TJ, Appleby PN, Reeves GK, Roddam AW, Helzlsouer KJ, Alberg AJ, et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer. 2011;105:709–22.
Kaaks R, Rinaldi S, Key TJ, Berrino F, Peeters PHM, Biessy C, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr-Relat Cancer. 2005;12:1071–82.
Zhang XH, Tworoger SS, Eliassen AH, Hankinson SE. Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res Treat. 2013;137:883–92.
Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–26.
Mesalic L, Tupkovic E, Kendic S, Balic D. Correlation between hormonal and lipid status in women in menopause. Bosn J Basic Med Sci. 2008;8:188–92.
Palmisano BT, Zhu L, Stafford JM. Role of estrogens in the regulation of liver lipid metabolism. Adv Exp Med Biol. 2017;1043:227–56.
Faulds MH, Zhao CY, Dahlman-Wright K, Gustafsson JA. The diversity of sex steroid action: regulation of metabolism by estrogen signaling. J Endocrinol. 2012;212:3–12.
Chlebowski RT, Anderson GL, Aragaki AK, Manson JE, Stefanick ML, Pan K, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the women’s health initiative randomized clinical trials. J Am Med Assoc. 2020;324:369–80.
Roman M, Sakshaug S, Graff-Iversen S, Vangen S, Weiderpass E, Ursin G, et al. Postmenopausal hormone therapy and the risk of breast cancer in Norway. Int J Cancer. 2016;138:584–93.
Ivanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN. Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxidative Med Cell Longevity. 2017;2017.
Pardhe BD, Ghimire S, Shakya J, Pathak S, Shakya S, Bhetwal A, et al. Elevated cardiovascular risks among postmenopausal women: a community based case control study from Nepal. Biochem Res Int. 2017;2017:3824903.
Shenoy R, Vernekar P. Fasting lipid profile in pre- and post-menopausal women: a prospective study. Int J Sci Study. 2015;3:116–119.
Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–11.
Alsaker MD, Janszky I, Opdahl S, Vatten LJ, Romundstad PR. Weight change in adulthood and risk of postmenopausal breast cancer: the HUNT study of Norway. Br J Cancer. 2013;109:1310–7.
Liu K, Zhang WN, Dai ZM, Wang M, Tian T, Liu XH, et al. Association between body mass index and breast cancer risk: evidence based on a dose-response meta-analysis. Cancer Manag Res. 2018;10:143–50.
Johnson KE, Siewert KM, Klarin D, Damrauer SM, Chang KM, Tsao PS, et al. The relationship between circulating lipids and breast cancer risk: a Mendelian randomization study. PLoS Med. 2020;17:e1003302.
Nowak C, Ärnlöv J. A Mendelian randomization study of the effects of blood lipids on breast cancer risk. Nat Commun. 2018;9:3957.
Beeghly-Fadiel A, Khankari NK, Delahanty RJ, Shu X-O, Lu Y, Schmidt MK, et al. A Mendelian randomization analysis of circulating lipid traits and breast cancer risk. Int J Epidemiol. 2019;49:1117–31.
Lecuyer L, Dalle C, Lefevre-Arbogast S, Micheau P, Lyan B, Rossary A, et al. Diet-related metabolomic signature of long-term breast cancer risk using penalized regression: an exploratory study in the SU.VI.MAX cohort. Cancer Epidemiol Biomark Prev. 2020;29:396–405.
Jobard E, Dossus L, Baglietto L, Fornili M, Lécuyer L, Mancini FR, et al. Investigation of circulating metabolites associated with breast cancer risk by untargeted metabolomics: a case–control study nested within the French E3N cohort. Br J Cancer. 2021;124:1734–43.
Craig A, Cloarec O, Holmes E, Nicholson JK, Lindon JC. Scaling and normalization effects in NMR spectroscopic metabonomic data sets. Anal Chem. 2006;78:2262–7.
Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–53.
Acknowledgements
The Trøndelag Health Study (HUNT) is a collaboration between HUNT Research Centre (Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology NTNU), Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health. The NMR analyses were performed at the MR Core Facility, Norwegian University of Science and Technology (NTNU), funded by the Faculty of Medicine at NTNU and Central Norway Regional Health Authority, and at Bruker BioSpin GmbH, Germany. HS, FF, CC and MS are employed at Bruker BioSpin. Bruker BioSpin has funded the release of the samples from the HUNT biobank.
Funding
This work has been supported by the Norwegian Financial Mechanism (2014-2021, JD, TFB, Project 2019/34/H/NZ7/00503) the Norwegian Cancer Society (GFG, 6834362 and 202021); Stiftelsen DAM (FW, 2020/FO298770); The K.G. Jebsen Foundation, the Liaison Committee for education, research and innovation in Central Norway (FW, 2020/3806-4), and the Joint Research Committee between St. Olavs hospital and the Faculty of Medicine and Health Sciences, NTNU (GFG, 28328 and TFB, 28346).
Author information
Authors and Affiliations
Contributions
Conceptualisation: JD, TFB and GFG. Data curation: JD, TFB and GFG. Formal analysis: JD, TA, TFB and GFG. Funding acquisition: TFB and GFG. Investigation: JD, HS, TA, FW, FF, CC and GFG. Methodology: JD, HS, TA, MS, TFB and GFG. Software: HS and MS. Supervision: TFB and GFG. Visualisation: JD. Writing—original draft: JD. Writing—review and editing: JD, HS, TA, FW, FF, CC, MS, TFB and GFG.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All participants have completed a written informed consent form, and the study was approved by the Ethics Committee of Central Norway (REK numbers #1995/8395 and #2017/2231).
Consent to publish
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Debik, J., Schäfer, H., Andreassen, T. et al. Lipoprotein and metabolite associations to breast cancer risk in the HUNT2 study. Br J Cancer 127, 1515–1524 (2022). https://doi.org/10.1038/s41416-022-01924-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01924-1
This article is cited by
-
Nuclear magnetic resonance-determined lipoprotein profile and risk of breast cancer: a Mendelian randomization study
Breast Cancer Research and Treatment (2023)