Abstract
Background
We investigate the current knowledge on circulating tumour DNA (ctDNA) and its clinical utility in predicting outcomes in patients with metastatic colorectal cancer (mCRC).
Methods
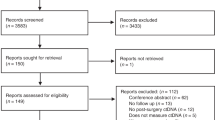
PubMed, Embase, Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials were searched. Last search 16/12/2020. We included studies on patients with mCRC reporting the predictive or prognostic value of ctDNA. We performed separate random-effects meta-analyses to investigate if baseline ctDNA and early changes in ctDNA levels during treatment were associated with survival. The risk of bias was assessed according to the Quality in Prognosis Studies tool.
Results
Seventy-one studies were included with 6930 patients. Twenty-four studies were included in meta-analyses. High baseline ctDNA level was associated with short progression-free survival (PFS) (HR = 2.2; 95% CI 1.8–2.8; n = 509) and overall survival (OS) (HR = 2.4; 95% CI 1.9–3.1; n = 1336). A small or no early decrease in ctDNA levels during treatment was associated with short PFS (HR = 3.0; 95% CI 2.2–4.2; n = 479) and OS (HR = 2.8; 95% CI 2.1–3.9; n = 583). Results on clonal evolution and lead-time were inconsistent. A majority of included studies (n = 50/71) had high risk of bias in at least one domain.
Conclusions
Plasma ctDNA is a strong prognostic biomarker in mCRC. However, true clinical utility is lacking.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The full text of all included studies were retrieved from the online databases PubMed, Embase, Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials. The data of this systematic review and meta-analyses are all public and available from PubMed, Embase, Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials. Template data collection forms, data extracted from included studies, data used for analyses and analytic code used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, et al. Global, regional, and national age-sex specifc mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–210.
Mandel P, Metais P. Les acides nucléiques du plasma sanguin chez l’homme. C R Seances Soc Biol Fil. 1948;142:241–3.
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422.
Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29:44–70.
Sepulveda AR, Hamilton SR, Allegra CJ, Grody W, Cushman-Vokoun AM, Funkhouser WK, et al. Molecular biomarkers for the evaluation of colorectal cancer: Guideline from the American society for clinical pathology, college of American pathologists, association for molecular pathology, and American society of clinical oncology. Arch Pathol Lab Med. 2017;141:625–57.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427–37. Mar 21
Lefebure B, Charbonnier F, Di Fiore F, Tuech JJ, Le Pessot F, Michot F, et al. Prognostic value of circulating mutant DNA in unresectable metastatic colorectal cancer. Ann Surg. 2010;251:275–80.
Spindler KLG, Pallisgaard N, Vogelius I, Jakobsen A. Quantitative cell-free DNA, KRAS, and BRAF mutations in plasma from patients with metastatic colorectal cancer during treatment with cetuximab and irinotecan. Clin Cancer Res. 2012;18:1177–85.
Vidal J, Muinelo L, Dalmases A, Jones F, Edelstein D, Iglesias M, et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann Oncol. 2017;28:1325–32.
Garlan F, Laurent-Puig P, Sefrioui D, Siauve N, Didelot A, Sarafan-Vasseur N, et al. Early evaluation of circulating tumor DNA as marker of therapeutic efficacy in metastatic colorectal cancer patients (PLACOL study). Clin Cancer Res. 2017;23:5416–25.
Yao J, Zang W, Ge Y, Weygant N, Yu P, Li L, et al. RAS/BRAF circulating tumor DNA mutations as a predictor of response to first-line chemotherapy in metastatic colorectal cancer patients. Can J Gastroenterol Hepatol. 2018;2018:4248971.
Thomsen CB, Hansen TF, Andersen RF, Lindebjerg J, Jensen LH, Jakobsen A. Monitoring the effect of first line treatment in RAS/RAF mutated metastatic colorectal cancer by serial analysis of tumor specific DNA in plasma. J Exp Clin Cancer Res. 2018;37:55.
Kim TW, Peeters M, Thomas A, Gibbs P, Hool K, Zhang J, et al. Impact of emergent circulating tumor DNA RAS mutation in panitumumab-treated chemoresistant metastatic colorectal cancer. Clin Cancer Res. 2018;24:5602–9.
Khan KH, Cunningham D, Werner B, Vlachogiannis G, Spiteri I, Heide T, et al. Longitudinal liquid biopsy and mathematical modeling of clonal evolution forecast time to treatment failure in the PROSPECT-C phase II colorectal cancer clinical trial. Cancer Discov. 2018;8:1270–85.
Takayama Y, Suzuki K, Muto Y, Ichida K, Fukui T, Kakizawa N, et al. Monitoring circulating tumor DNA revealed dynamic changes in KRAS status in patients with metastatic colorectal cancer. Oncotarget. 2018;9:24398–413.
Maurel J, Alonso V, Escudero P, Fernández-Martos C, Salud A, Méndez M, et al. Clinical Impact of circulating tumor RAS and BRAF mutation dynamics in patients with metastatic colorectal cancer treated with first-line chemotherapy plus anti–epidermal growth factor receptor therapy. JCO Precis Oncol. 2019;3:1–16.
Cremolini C, Rossini D, Dell’Aquila E, Lonardi S, Conca E, Del Re M, et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 2019;5:343–50.
Elez E, Chianese C, Sanz-García E, Martinelli E, Noguerido A, Mancuso FM, et al. Impact of circulating tumor DNA mutant allele fraction on prognosis in RAS-mutant metastatic colorectal cancer. Mol Oncol. 2019;13:1827–35.
Spindler KG, Appelt AL, Pallisgaard N, Andersen RF, Jakobsen A. KRAS-mutated plasma DNA as predictor of outcome from irinotecan monotherapy in metastatic colorectal cancer. Br J Cancer. 2013;109:3067–72.
Amatu A, Schirripa M, Tosi F, Lonardi S, Bencardino K, Bonazzina E, et al. High circulating methylated DNA is a negative predictive and prognostic marker in metastatic colorectal cancer patients treated with regorafenib. Front Oncol. 2019;9:622.
Jensen LH, Olesen R, Petersen LN, Boysen AK, Andersen RF, Lindebjerg J, et al. NPY Gene methylation as a universal, longitudinal plasma marker for evaluating the clinical benefit from last-line treatment with regorafenib in metastatic colorectal cancer. Cancers (Basel). 2019;11:1649.
Siravegna G, Sartore-Bianchi A, Nagy RJ, Raghav K, Odegaard JI, Lanman RB, et al. Plasma HER2 (ERBB2) copy number predicts response to HER2-targeted therapy in metastatic colorectal cancer. Clin Cancer Res J Am Assoc Cancer Res. 2019;25:3046–53.
Holm M, Andersson E, Osterlund E, Ovissi A, Soveri L-MM, Anttonen A-KK, et al. Detection of KRAS mutations in liquid biopsies from metastatic colorectal cancer patients using droplet digital PCR, Idylla, and next generation sequencing. PLoS ONE. 2020;15:e0239819.
Thomsen CB, Hansen TF, Andersen RF, Lindebjerg J, Jensen LH, Jakobsen A. Early identification of treatment benefit by methylated circulating tumor DNA in metastatic colorectal cancer. Ther Adv Med Oncol. 2020;12:1758835920918472.
Max Ma X, Bendell JC, Hurwitz HI, Ju C, Lee JJ, Lovejoy A, et al. Disease monitoring using post-induction circulating tumor DNA analysis following first-line therapy in patients with metastatic colorectal cancer. Clin cancer Res. 2020;26:4010–7.
Lueong SS, Herbst A, Liffers S-T, Bielefeld N, Horn PA, Tannapfel A, et al. Serial Circulating tumor DNA mutational status in patients with KRAS-mutant metastatic colorectal cancer from the phase 3 AIO KRK0207 trial. Clin Chem. 2020;66:1510–20.
Bouchahda M, Saffroy R, Karaboue A, Hamelin J, Innominato P, Saliba F, et al. Undetectable RAS-mutant clones in plasma: possible implication for anti-EGFR therapy and prognosis in patients with RAS-mutant metastatic colorectal cancer. JCO Precis Oncol. 2020;4:1070–1079.
Yamada T, Matsuda A, Takahashi G, Iwai T, Takeda K, Ueda K, et al. Emerging RAS, BRAF, and EGFR mutations in cell-free DNA of metastatic colorectal patients are associated with both primary and secondary resistance to first-line anti-EGFR therapy. Int J Clin Oncol. 2020;25:1523–32.
Yu S, Nakamura M, Ishizaki M, Kataoka M, Satake H, Kitazono M, et al. RAS Mutations in circulating tumor DNA and clinical outcomes of rechallenge treatment with anti-EGFR antibodies in patients with metastatic colorectal cancer. JCO Precis Oncol. 2020;4:898–911.
Sefrioui D, Sarafan-Vasseur N, Beaussire L, Baretti M, Gangloff A, Blanchard F, et al. Clinical value of chip-based digital-PCR platform for the detection of circulating DNA in metastatic colorectal cancer. Dig Liver Dis. 2015;47:884–90.
Jacobs SA, Lee JJ, George TJ, Wade JL, Stella PJ, Wang D, et al. Neratinib plus Cetuximab in quadruple WT (KRAS, NRAS, BRAF, PIK3CA) metastatic colorectal cancer resistant to cetuximab or panitumumab: NSABP FC-7, A Phase Ib Study. Clin Cancer Res. 2020;27:1612–22.
Unseld M, Belic J, Pierer K, Zhou Q, Moser T, Bauer R, et al. A higher ctDNA fraction decreases survival in regorafenib-treated metastatic colorectal cancer patients. Results from the regorafenib’s liquid biopsy translational biomarker phase II pilot study. Int J Cancer. 2020;148:1452–61.
Kang J-K, Heo S, Kim H-P, Song S-H, Yun H, Han S-W, et al. Liquid biopsy-based tumor profiling for metastatic colorectal cancer patients with ultra-deep targeted sequencing. PLoS ONE. 2020;15:e0232754.
Lyskjær I, Kronborg CS, Rasmussen MH, Sørensen BS, Demuth C, Rosenkilde M, et al. Correlation between early dynamics in circulating tumour DNA and outcome from FOLFIRI treatment in metastatic colorectal cancer. Sci Rep. 2019;9:11542.
Wong AL, Lim JS, Sinha A, Gopinathan A, Lim R, Tan CS, et al. Tumour pharmacodynamics and circulating cell free DNA in patients with refractory colorectal carcinoma treated with regorafenib. J Transl Med. 2015;13:57. 2015/04/19
Shitara K, Yonesaka K, Denda T, Yamazaki K, Moriwaki T, Tsuda M, et al. Randomized study of FOLFIRI plus either panitumumab or bevacizumab for wild-type KRAS colorectal cancer-WJOG 6210G. Cancer Sci. 2016;107:1843–50.
Yamada T, Iwai T, Takahashi G, Kan H, Koizumi M, Matsuda A, et al. Utility of KRAS mutation detection using circulating cell-free DNA from patients with colorectal cancer. Cancer Sci. 2016;107:936–43.
El Messaoudi S, Mouliere F, Du Manoir S, Bascoul-Mollevi C, Gillet B, Nouaille M, et al. Circulating DNA as a strong multimarker prognostic tool for metastatic colorectal cancer patient management care. Clin Cancer Res. 2016;22:3067–77.
Herbst A, Vdovin N, Gacesa S, Ofner A, Philipp A, Nagel D, et al. Methylated free-circulating HPP1 DNA is an early response marker in patients with metastatic colorectal cancer. Int J Cancer. 2017;140:2134–44.
Toledo RA, Cubillo A, Vega E, Garralda E, Alvarez R, de la Varga LU, et al. Clinical validation of prospective liquid biopsy monitoring in patients with wild-type RAS metastatic colorectal cancer treated with FOLFIRI-cetuximab. Oncotarget. 2017;8:35289–300.
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK). Eur J Cancer. 2005;41:1690–6.
Khan K, Rata M, Cunningham D, Koh DM, Tunariu N, Hahne JC, et al. Functional imaging and circulating biomarkers of response to regorafenib in treatment-refractory metastatic colorectal cancer patients in a prospective phase II study. Gut. 2018;67:1484–92.
Boeckx N, Op de Beeck K, Beyens M, Deschoolmeester V, Hermans C, De Clercq P, et al. Mutation and methylation analysis of circulating tumor DNA can be used for follow-up of metastatic colorectal cancer patients. Clin Color Cancer. 2018;17:e369–79.
Hsu HC, Lapke N, Wang CW, Lin PY, You JF, Yeh CY, et al. Targeted sequencing of circulating tumor DNA to monitor genetic variants and therapeutic response in metastatic colorectal cancer. Mol Cancer Ther. 2018;17:2238–47.
Vandeputte C, Kehagias P, El Housni H, Ameye L, Laes JF, Desmedt C, et al. Circulating tumor DNA in early response assessment and monitoring of advanced colorectal cancer treated with a multi-kinase inhibitor. Oncotarget. 2018;9:17756–69.
Corcoran RB, Andre T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAF(V600E)-mutant colorectal cancer. Cancer Discov. 2018;8:428–43.
Barault L, Amatu A, Siravegna G, Ponzetti A, Moran S, Cassingena A, et al. Discovery of methylated circulating DNA biomarkers for comprehensive non-invasive monitoring of treatment response in metastatic colorectal cancer. Gut. 2018;67:1995–2005.
Jia N, Sun Z, Gao X, Cheng Y, Zhou Y, Shen C, et al. Serial monitoring of circulating tumor DNA in patients with metastatic colorectal cancer to predict the therapeutic response. Front Genet. 2019;10:470.
Osumi H, Shinozaki E, Yamaguchi K, Zembutsu H. Early change in circulating tumor DNA as a potential predictor of response to chemotherapy in patients with metastatic colorectal cancer. Sci Rep. 2019;9:17358.
Moser T, Waldispuehl-Geigl J, Belic J, Weber S, Zhou Q, Hasenleithner SO, et al. On-treatment measurements of circulating tumor DNA during FOLFOX therapy in patients with colorectal cancer. npj Precis Oncol. 2020;4:30.
Klein-Scory S, Wahner I, Maslova M, Al-Sewaidi Y, Pohl M, Mika T, et al. Evolution of RAS mutational status in liquid biopsies during first-line chemotherapy for metastatic colorectal cancer. Front Oncol. 2020;10:1115.
Parikh AR, Mojtahed A, Schneider JL, Kanter K, Van Seventer EE, Fetter IJ, et al. Serial ctDNA monitoring to predict response to systemic therapy in metastatic gastrointestinal cancers. Clin Cancer Res. 2020;26:1877–85.
Wang C, Chevalier D, Saluja J, Sandhu J, Lau C, Fakih M. Regorafenib and nivolumab or pembrolizumab combination and circulating tumor dna response assessment in refractory microsatellite stable colorectal cancer. Oncologist. 2020;25:e1188–94.
Tie J, Kinde I, Wang Y, Wong HL, Roebert J, Christie M, et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol. 2015;26:1715–22.
Hong DS, Morris VK, El Osta B, Sorokin AV, Janku F, Fu S, et al. Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with BRAFV600E mutation. Cancer Discov. 2016;6:1352–65.
Yamauchi M, Urabe Y, Ono A, Miki D, Ochi H, Chayama K. Serial profiling of circulating tumor DNA for optimization of anti-VEGF chemotherapy in metastatic colorectal cancer patients. Int J Cancer. 2018;142:1418–26.
Zou D, Day R, Cocadiz JA, Parackal S, Mitchell W, Black MA, et al. Circulating tumor DNA is a sensitive marker for routine monitoring of treatment response in advanced colorectal cancer. Carcinogenesis. 2020;41:1507–17.
Xu JM, Wang YLYL, Liu T, Ni M, Li MS, Lin L, et al. PIK3CA mutations contribute to acquired cetuximab resistance in patients with metastatic colorectal cancer. Clin Cancer Res. 2017;23:4602–16.
Liu R, Zhao X, Guo W, Huang M, Qiu L, Zhang W, et al. Dynamic monitoring of HER2 amplification in circulating DNA of patients with metastatic colorectal cancer treated with cetuximab. Clin Transl Oncol. 2020;22:928–34.
Siena S, Sartore-Bianchi A, Garcia-Carbonero R, Karthaus M, Smith D, Tabernero J, et al. Dynamic molecular analysis and clinical correlates of tumor evolution within a phase II trial of panitumumab-based therapy in metastatic colorectal cancer. Ann Oncol. 2018;29:119–26.
Choi IS, Kato S, Fanta PT, Leichman L, Okamura R, Raymond VM, et al. Genomic profiling of blood-derived circulating tumor DNA from patients with colorectal cancer: Implications for response and resistance to targeted therapeutics. Mol Cancer Ther. 2019;18:1852–62.
Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21:795–801.
Zhang H, Liu R, Yan C, Liu L, Tong Z, Jiang W, et al. Advantage of next-generation sequencing in dynamic monitoring of circulating tumor DNA over droplet digital PCR in cetuximab treated colorectal cancer patients. Transl Oncol. 2019;12:426–31.
Thierry AR, Pastor B, Jiang ZQ, Katsiampoura AD, Parseghian C, Loree JM, et al. Circulating DNA demonstrates convergent evolution and common resistance mechanisms during treatment of colorectal cancer. Clin Cancer Res. 2017;23:4578–91.
Thomsen CB, Andersen RF, Lindebjerg J, Hansen TF, Jensen LH, Jakobsen A. Plasma dynamics of RAS/RAF mutations in patients with metastatic colorectal cancer receiving chemotherapy and anti-EGFR treatment. Clin Colorectal Cancer. 2019;18:28–33.
van Helden EJ, Angus L, Menke-van der Houven van Oordt CW, Heideman DAMM, Boon E, van Es SC, et al. RAS and BRAF mutations in cell-free DNA are predictive for outcome of cetuximab monotherapy in patients with tissue-tested RAS wild-type advanced colorectal cancer. Mol Oncol. 2019;13:2361–74.
Shitara K, Yamanaka T, Denda T, Tsuji Y, Shinozaki K, Komatsu Y, et al. REVERCE: a randomized phase II study of regorafenib followed by cetuximab versus the reverse sequence for previously treated metastatic colorectal cancer patients. Ann Oncol J Eur Soc Med Oncol. 2019;30:259–65.
Van Emburgh BO, Arena S, Siravegna G, Lazzari L, Crisafulli G, Corti G, et al. Acquired RAS or EGFR mutations and duration of response to EGFR blockade in colorectal cancer. Nat Commun. 2016;7:13665.
Ballman KV. Biomarker: predictive or prognostic? J Clin Oncol. 2015;33:3968–71.
International Organization for Standardization—ISO 20186-3:2019.—Molecular in vitro diagnostic examinations—Specifications for pre-examination processes for venous whole blood—Part 3: Isolated circulating cell free DNA from plasma [Internet]. 2022. https://www.iso.org/standard/69800.html.
Johansson G, Andersson D, Filges S, Li J, Muth A, Godfrey TE, et al. Considerations and quality controls when analyzing cell-free tumor DNA. Biomol Detect Quantif. 2019;17:100078.
Demuth C, Spindler KLG, Johansen JS, Pallisgaard N, Nielsen D, Hogdall E, et al. Measuring KRAS mutations in circulating tumor DNA by droplet digital PCR and next-generation sequencing. Transl Oncol. 2018;11:1220–4.
Peeters M, Price T, Boedigheimer M, Kim TW, Ruff P, Gibbs P, et al. Evaluation of emergent mutations in circulating cell-free DNA and clinical outcomes in patients with metastatic colorectal cancer treated with panitumumab in the ASPECCT study. Clin Cancer Res J Am Assoc Cancer Res. 2019;25:1216–25.
Acknowledgements
We would like to thank Marie Susanna Isachsen, Senior Librarian, University of Oslo for help in developing the search string. Additionally, we would like to thank Lene Kristine Juvet, Scientific Director, Norwegian Institute of Public Health and Kjell M. Tveit, Professor Emeritus, University of Oslo for valuable scientific discussions.
Funding
LC was supported by DCCC ctDNA Research Center—The Danish Research Center for Circulating Tumor DNA Guided Cancer Management, Danish Cancer Society (grant no. R257-A14700) and Danish Comprehensive Cancer Centers. KLS was supported by Health Research Foundation of Central Denmark Region (grant no. A1602). Funding has been provided by Danish Cancer Society, Health Research Foundation of Central Denmark Region. Registered PROSPERO(CRD42019125630). The remaining authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Study concept and design: JH, AB, KLS, LC, data collection and collation: JH, AB, LC, statistical analysis: JH, LC, writing—original draft: JH, LC, writing—review and editing: JH, AB, NP, TKG, EHK, KLS, LC, Interpretation of the data, critical revision of the paper for important intellectual content and approval of the final paper for submission: JH, AB, NP, TKG, EHK, KLS, LC. The corresponding author proves that all listed authors meet the authorship criteria, and that no other eligible authors have been omitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Callesen, L.B., Hamfjord, J., Boysen, A.K. et al. Circulating tumour DNA and its clinical utility in predicting treatment response or survival in patients with metastatic colorectal cancer: a systematic review and meta-analysis. Br J Cancer 127, 500–513 (2022). https://doi.org/10.1038/s41416-022-01816-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01816-4
This article is cited by
-
Circulating DNA and frequency of colorectal cancer brain metastases in a presumed high-risk group
Scientific Reports (2023)
-
BRAFV600E Metastatic Colorectal Cancer: Perspective from a Patient, a Caregiver, and an Oncologist
Advances in Therapy (2023)
-
Cell-free circulating RAS mutation concentrations significantly impact the survival of metastatic colorectal cancer patients
Journal of Cancer Research and Clinical Oncology (2023)