Abstract
Background
Aflibercept is an antiangiogenic drug against metastatic colorectal cancer (mCRC) combined with 5-fluorouracil/leucovorin/irinotecan (FOLFIRI); however, no antiangiogenic biomarker has yet been validated. We assessed aflibercept plus FOLFIRI, investigating the biomarker role of baseline vascular endothelial growth factor A (VEGF-A) and angiotensin-converting enzyme (ACE).
Methods
Phase II trial in oxaliplatin-treated mCRC patients who received aflibercept plus FOLFIRI. The reported 135 ng/mL ACE cut-off was used and ROC analysis was performed to assess the optimal VEGF-A cut-off for progression-free survival (PFS). Overall survival (OS), time to progression (TTP), time to treatment failure (TTF), overall response rate (ORR) and disease control rate (DCR) were also assessed.
Results
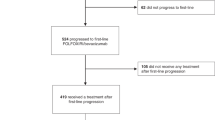
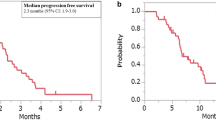
In total, 101 patients were followed for a median of 12 (6–17) months. The 1941 pg/mL VEGF-A was an optimal cut-off, with a longer median PFS when VEGF-A was <1941 versus ≥1941 pg/mL (9 versus 4 months). Patients with VEGF-A < 1941 pg/mL showed longer median OS (19 versus 8 months), TTP (9 versus 4 months) and TTF (8 versus 4 months), along with higher ORR (26% versus 9%) and DCR (81% versus 55%). No differences were identified according to ACE levels.
Conclusions
This study supports aflibercept plus FOLFIRI benefits, suggesting VEGF-A as a potential biomarker to predict better outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data relevant to the study are included in the article or uploaded as supplementary information. Further data are available from the authors upon reasonable request and with permission of the Spanish Cooperative Group for the Treatment of Digestive Tumours (TTD).
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
American Cancer Society. Colorectal cancer facts & figures 2017–2019. Atlanta: American Cancer Society; 2017.
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422. https://doi.org/10.1093/annonc/mdw235.
NCCN. NCCN clinical practice guidelines in Oncology (NCCN Guidelines). Colon cancer. Version 3.2019, https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf, Accessed October 28, 2019 (2019).
Stanel SC, Sjoberg J, Salmonson T, Foggi P, Caleno M, Melchiorri D, et al. European Medicines Agency approval summary: Zaltrap for the treatment of patients with oxaliplatin-resistant metastatic colorectal cancer. ESMO Open. 2017;2:e000190. https://doi.org/10.1136/esmoopen-2017-000190.
Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–506. https://doi.org/10.1200/JCO.2012.42.8201.
Tabernero J, Van Cutsem E, Lakomy R, Prausova J, Ruff P, van Hazel GA, et al. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: prespecified subgroup analyses from the VELOUR trial. Eur J Cancer. 2014;50:320–31. https://doi.org/10.1016/j.ejca.2013.09.013.
Van Cutsem E, Joulain F, Hoff PM, Mitchell E, Ruff P, Lakomy R, et al. Aflibercept plus FOLFIRI vs. placebo plus FOLFIRI in second-line metastatic colorectal cancer: a post hoc analysis of survival from the phase III VELOUR study subsequent to exclusion of patients who had recurrence during or within 6 months of completing adjuvant oxaliplatin-based therapy. Target Oncol. 2016;11:383–400. https://doi.org/10.1007/s11523-015-0402-9.
Van Cutsem E, Paccard C, Chiron M, Tabernero J. Impact of prior bevacizumab treatment on VEGF-A and PlGF levels and outcome following second-line aflibercept treatment: Biomarker post hoc analysis of the VELOUR trial. Clin Cancer Res. 2020;26:717–25. https://doi.org/10.1158/1078-0432.CCR-19-1985.
Moreno-Munoz D, de la Haba-Rodriguez JR, Conde F, Lopez-Sanchez LM, Valverde A, Hernandez V, et al. Genetic variants in the renin-angiotensin system predict response to bevacizumab in cancer patients. Eur J Clin Investig. 2015;45:1325–32. https://doi.org/10.1111/eci.12557.
Fernandez Montes A, Martinez Lago N, Covela Rua M, de la Camara Gomez J, Gonzalez Villaroel P, Mendez Mendez JC, et al. Efficacy and safety of FOLFIRI/aflibercept in second-line treatment of metastatic colorectal cancer in a real-world population: prognostic and predictive markers. Cancer Med. 2019;8:882–9. https://doi.org/10.1002/cam4.1903.
Feliu J, Diez de Corcuera I, Manzano JL, Valladares-Ayerbes M, Alcaide J, Garcia Garcia T, et al. Effectiveness and safety of aflibercept for metastatic colorectal cancer: retrospective review within an early access program in Spain. Clin Transl Oncol. 2017;19:498–507. https://doi.org/10.1007/s12094-016-1556-3.
Hofheinz R, Scholten F, Derigs H, Thaler J, von Moos R. PD-026Quality of life in patients treated with aflibercept and FOLFIRI for metastatic colorectal cancer: interim analysis with focus on therapy lines of the non-interventional study QoLiTrap (AIO-LQ-0113). Ann Oncol. 2019;30:iv118. https://doi.org/10.1093/annonc/mdz156.025.
Vera R, Mata E, Gonzalez E, Juez I, Alonso V, Iranzo P, et al. Is aflibercept an optimal treatment for wt RAS mCRC patients after progression to first line containing anti-EGFR? Int J Colorectal Dis. 2020;35:739–46. https://doi.org/10.1007/s00384-020-03509-x.
Wirapati P, Pomella V, Vandenbosch B, Kerr P, Maiello E, Jeffery GM, et al. Velour trial biomarkers update: impact of RAS, BRAF, and sidedness on aflibercept activity. J Clin Oncol. 2017;35:3538–3538. https://doi.org/10.1200/JCO.2017.35.15_suppl.3538.
Marisi G, Scarpi E, Passardi A, Nanni O, Ragazzini A, Valgiusti M, et al. Circulating VEGF and eNOS variations as predictors of outcome in metastatic colorectal cancer patients receiving bevacizumab. Sci Rep. 2017;7:1293. https://doi.org/10.1038/s41598-017-01420-0.
Hayashi H, Arao T, Matsumoto K, Kimura H, Togashi Y, Hirashima Y, et al. Biomarkers of reactive resistance and early disease progression during chemotherapy plus bevacizumab treatment for colorectal carcinoma. Oncotarget. 2014;5:2588–95. https://doi.org/10.18632/oncotarget.1811.
Willett CG, Duda DG, di Tomaso E, Boucher Y, Ancukiewicz M, Sahani DV, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol. 2009;27:3020–6. https://doi.org/10.1200/JCO.2008.21.1771.
Alidzanovic L, Starlinger P, Schauer D, Maier T, Feldman A, Buchberger E, et al. The VEGF rise in blood of bevacizumab patients is not based on tumor escape but a host-blockade of VEGF clearance. Oncotarget. 2016;7:57197–212. https://doi.org/10.18632/oncotarget.11084.
Acknowledgements
The authors would like to thank participating patients and both medical and nursing staff involved in the conduct of the study. The authors would also like to acknowledge the Spanish Cooperative Group for the Treatment of Digestive Tumours (TTD) for sponsoring the study (funded by Sanofi) and the following specific people for their cooperation and support: (1) Study chairs: E. Aranda and M.A. Gómez-España (Hospital Reina Sofía, Córdoba, Spain). (2) Spanish Cooperative Group for the Treatment of Digestive Tumours (TTD): I. Ruiz de Mena and S. Rodríguez. The medical writing support was provided by Esther Álvarez-García, DVM, PhD, at Dynamic Science S.L. (Madrid, Spain) during the preparation of this paper, funded by the Spanish Cooperative Group for the Treatment of Digestive Tumours (TTD; Madrid, Spain). The medical writing support included drafting the article, copyediting, editorial assistance and production assistance.
Funding
This work was supported by the Spanish Cooperative Group for the Treatment of Digestive Tumours (TTD) through an unrestricted grant provided by Sanofi (no grant number is applicable).
Author information
Authors and Affiliations
Contributions
EA and MAGE contributed to the study design. All authors contributed to the acquisition, analysis, and/or interpretation of data. All authors revised critically the manuscript for important intellectual content, approved the version to be published and are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
MAGE reports other from ROCHE, other from MYLAN, other from SERVIER, other from AMGEN, other from MERCK, other from BAYER, outside the submitted work. MJOM reports personal fees and non-financial support from ROCHE, personal fees and non-financial support from AMGEN, personal fees from SERVIER, personal fees from SANOFI, outside the submitted work. MVA reports grants and personal fees from ROCHE, personal fees from MERCK, personal fees from AMGEN, personal fees from SANOFI, during the conduct of the study; personal fees from SERVIER, personal fees from BAYER, personal fees from CELGENE, outside the submitted work. MS reports grants from AMGEN, grants from MERCK, grants from CELGENE, grants from SERVIER, grants from ROVI, grants from BMS, grants from LEOPHARMA, grants from ROCHE, grants from MYLAN, grants from KHERM PHARMA, outside the submitted work. EMC reports having acted as a speaker for SANOFI. JT reports personal financial interest in form of scientific consultancy role for Array Biopharma, AstraZeneca, Avvinity, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Ikena Oncology, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, Neophore, Novartis, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Seattle Genetics, Scandion Oncology, Servier, Taiho, Tessa Therapeutics and TheraMyc. And also the educational collaboration with Imedex, Medscape Education, MJH Life Sciences, PeerView Institute for Medical Education and Physicians Education Resource (PER). JT also declares institutional financial interest in form of financial support for clinical trials or contracted research for Amgen Inc, Array Biopharma Inc, AstraZeneca Pharmaceuticals LP, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Debiopharm International SA, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Janssen-Cilag SA, MedImmune, Menarini, Merck Health KGAA, Merck Sharp & Dohme, Merus NV, Mirati, Novartis Farmacéutica SA, Pfizer, Pharma Mar, Sanofi Aventis Recherche & Développement, Servier, Taiho Pharma USA Inc, Spanish Association Against Cancer Scientific Foundation and Cancer Research UK. MCR reports personal fees and non-financial support from ROCHE, non-financial support from AMGEN, personal fees from NOVARTIS, non-financial support from SERVIER, personal fees and non-financial support from BAYER, personal fees and non-financial support from MERCK, outside the submitted work. EA reports honoraria for the advisory roles from AMGEN, BAYER, CELGENE, MERCK, ROCHE, SANOFI. The remaining authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted according to the Helsinki Declaration and national regulations. It was approved by the ethics committee of Hospital Reina Sofía (Córdoba, Spain; approval number/ID: 255) and patients gave their written informed consent before taking part.
Consent to publish
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Élez, E., Gómez-España, M.A., Grávalos, C. et al. Effect of aflibercept plus FOLFIRI and potential efficacy biomarkers in patients with metastatic colorectal cancer: the POLAF trial. Br J Cancer 126, 874–880 (2022). https://doi.org/10.1038/s41416-021-01638-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01638-w