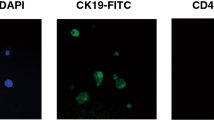
Abstract
Background
Circulating tumour cells (CTCs) can be used to monitor cancer longitudinally, but their use in non-small cell lung cancer (NSCLC) is limited due to low numbers in the peripheral blood. Through diagnostic leukapheresis (DLA) CTCs can be obtained from larger blood volumes.
Methods
Patients with all stages of NSCLC were selected. One total body blood volume was screened by DLA before and after treatment. Peripheral blood was drawn pre- and post DLA for CTC enumeration by CellSearch. CTCs were detected in the DLA product (volume equalling 2 × 108 leucocytes) and after leucocyte depletion (RosetteSep, 9 mL DLA product). Single-cell, whole-genome sequencing was performed on isolated CTCs.
Results
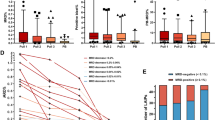
Fifty-six patients were included. Before treatment, CTCs were more often detected in DLA (32/55, 58%) than in the peripheral blood (pre-DLA: 18/55, 33%; post DLA: 13/55, 23%, both at p < 0.01). CTCs per 7.5 mL DLA product were median 9.2 times (interquartile range = 5.6–24.0) higher than CTCs in 7.5 mL blood. RosetteSEP did not significantly improve CTC detection (pretreatment: 34/55, 62%, post treatment: 16/34, 47%) and CTCs per mL even decreased compared to DLA (p = 0.04)..
Patients with advanced-stage disease with DLA-CTC after treatment showed fewer tumour responses and shorter progression-free survival (PFS) than those without DLA-CTC (median PFS, 2.0 vs 12.0 months, p < 0.01). DLA-CTC persistence after treatment was independent of clinical factors associated with shorter PFS (hazard ratio (HR) = 5.8, 95% confidence interval (CI), 1.4–35.5, p = 0.02). All evaluable CTCs showed aneuploidy.
Conclusions
DLA detected nine times more CTCs than in the peripheral blood. The sustained presence of CTCs in DLA after treatment was associated with therapy failure and shortened PFS.
Trial registration
The study was approved by the Medical Ethical Committee (NL55754.042.15) and was registered in the Dutch trial register (NL5423).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data obtained in this study are available upon reasonable request from the corresponding author.
References
Wit S de, Rossi E, Weber S, Tamminga M, Manicone M, Swennenhuis JF, et al. Single tube liquid biopsy for advanced non-small cell lung cancer. Int J Cancer. 2019;12:3127–37.
Wit Sde, Dalum Gvan, Lenferink ATM, Tibbe AGJ, Hiltermann TJN, Groen HJM. et al. The detection of EpCAM+ and EpCAM– circulating tumor cells. Sci Rep. 2015;5:12270–9. https://doi.org/10.1038/srep12270.
Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. 2012;30:525–32. https://doi.org/10.1200/JCO.2010.33.3716.
Krebs MG, Hou JM, Sloane R, Lanaschire L, Priest L, Nonaka D, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7:306–15. https://doi.org/10.1097/JTO.0b013e31823c5c16.
Punnoose EA, Atwal S, Liu W, Raja R, Fine BM, Hugher BGM, et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res. 2012;18:2391–401. https://doi.org/10.1158/1078-0432.CCR-11-3148.
Hofman V, Bonnetaud C, Ilie MI, Vielh P, Vielh P, Vignaud JM, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res. 2010;17:827–35. https://doi.org/10.1158/1078-0432.ccr-10-0445.
Tamminga M, Wit SD, Hiltermann TJN, Timens W, Schuuring E, Terstappen LWWM, et al. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J Immunother Cancer. 2019;2:1–9. https://doi.org/10.1186/s40425-019-0649-2.
Tamminga M, Wit SD, Schuuring E, Timens W, Terstappen LWMM, Hiltermann HJMG, et al. Circulating tumor cells in lung cancer are prognostic and predictive for worse tumor response in both targeted- and chemotherapy. TLCR. 2019;8:854–61. https://doi.org/10.21037/tlcr.2019.11.06.
Coumans FAW, Ligthart ST, Uhr JW, Terstappen LWMM. Challenges in the enumeration and phenotyping of CTC. Clin Cancer Res. 2012;18:5711–8. https://doi.org/10.1158/1078-0432.CCR-12-1585.
Fischer JC, Niederacher D, Topp SA, Honsich E, Schumacher S, Schmitz N, et al. Diagnostic leukapheresis enables reliable detection of circulating tumor cells of nonmetastatic cancer patients. Proc Natl Acad Sci USA. 2013;110:16580–5. https://doi.org/10.1073/pnas.1313594110.
Fehm TN, Meier‐Stiegen F, Driemel C, Jäger B, Reinhardt F, Naskou J. et al. Diagnostic leukapheresis for CTC analysis in breast cancer patients: CTC frequency, clinical experiences and recommendations for standardized reporting. Cytom Part A. 2018;93:1213–9. https://doi.org/10.1002/cyto.a.23669.
Andree KC, Mentink A, Zeune LL, Terstappen LWWM, Stoecklein NH, Neves RP, et al. Toward a real liquid biopsy in metastatic breast and prostate cancer: diagnostic leukApheresis increases CTC yields in a European prospective multicenter study (CTCTrap). Int J Cancer. 2018;143:2584–91. https://doi.org/10.1002/ijc.31752.
Andree KC, Van Dalum G, Terstappen LWMM. Challenges in circulating tumor cell detection by the CellSearch system. Mol Oncol. 2016;10:395–407. https://doi.org/10.1016/j.molonc.2015.12.002.
Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51;224–32.
Stoecklein NH, Fischer JC, Niederacher D, Terstappen LWMM. Challenges for CTC-based liquid biopsies: low CTC frequency and diagnostic leukapheresis as a potential solution. Expert Rev Mol Diagn. 2015;7159. https://doi.org/10.1586/14737159.2016.1123095.
Punzel M, Kozlova A, Quade A, Schmidt AH, Smith R. Evolution of MNC and lymphocyte collection settings employing different Spectra Optia ® Leukapheresis systems. Vox Sang. 2017;112:586–94. https://doi.org/10.1111/vox.12540.
Zeune LL, de Wit S, Berghuis AMS, IJzerman MJ, Terstappen LWMM, Brune C. How to agree on a CTC: evaluating the consensus in circulating tumor cellscoring. Cytom A. 2018;93:1202–6. https://doi.org/10.1002/cyto.a.23576.
Stevens M, Oomens L, Broekmaat J, Weersink J, Abali F, Swennenhuis JF, et al. VyCAP’s puncher technology for single cell identification, isolation, and analysis. Cytom Part A. 2018;93:1255–9. https://doi.org/10.1002/cyto.a.23631.
van den Bos H, Bakker B, Taudt A, Guryev V, Colomé-Tatché M, Lansdorp PM, et al. Quantification of aneuploidy in mammalian systems. New York: Humana Press; 2019. p. 159–90.
Bakker B, Taudt A, Belderbos ME, Porubsky D, Spierings DCJ, De Jong TV, et al. Single-cell sequencing reveals karyotype heterogeneity in murine and human malignancies. Genome Biol. 2016;17:115 https://doi.org/10.1186/s13059-016-0971-7.
Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Eng J Med. 2008;359;366–77. https://doi.org/10.1056/NEJMoa0800668.
Sundaresan TK, Sequist LV, Heymach JV, Riely GJ, Jänne PA, Koch WH, et al. Detection of T790M, the acquired resistance EGFR mutation, by tumor biopsy versus noninvasive blood-based analyses. Clin Cancer Res. 2015;1–9. https://doi.org/10.1158/1078-0432.CCR-15-1031.
Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, et al. Circulating tumor cells versus imaging—predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12:6403–9. https://doi.org/10.1158/1078-0432.CCR-05-1769.
Hiltermann TJN, Pore MM, van den Berg A, Timens W, Boezen HM, Liesker JJW, et al. Circulating tumor cells in small-cell lung cancer: A predictive and prognostic factor. Ann Oncol. 2012;23:2937–42. https://doi.org/10.1093/annonc/mds138.
Tamminga M, de Wit S, van de Wauwer C, Van den Bos H, Swennenhuis JF, Klinkenberg TJ, et al. Release of circulating tumor cells during surgery for non–small cell lung cancer: Are they what they appear to be? Clin Cancer Res. 2019;1–12. https://doi.org/10.1158/1078-0432.ccr-19-2541.
Chemi F, Rothwell DG, McGranahan N, Gulati A, Abbosh C, Pearce SP, et al. Pulmonary venous circulating tumor cell dissemination before tumor resection and disease relapse. Nat Med. 2019;25:1534–9. https://doi.org/10.1038/s41591-019-0593-1.
Lambros MB, Seed G, Sumanasuriya S, Gil V, Crespo M, Fontes M, et al. Single-cell analyses of prostate cancer liquid biopsies acquired by apheresis. Clin Cancer Res. 2018;24:5635–44. https://doi.org/10.1158/1078-0432.CCR-18-0862.
McLeod BC, Sniecinski I, Ciavarella D, Owen H, Price TH, Randels MJ, et al. Frequency of immediate adverse effects associated with therapeutic apheresis. Transfusion. 1999;39:282–8. https://doi.org/10.1046/j.1537-2995.1999.39399219285.
Crocco I, Franchini M, Garozzo G, Gandini AR, Gandini G, Bonomo P, et al. Adverse reactions in blood and apheresis donors: experience from two Italian transfusion centres. Blood Transfus. 2009;7:35 https://doi.org/10.2450/2008.0018-08.
Kraal KCJM, Timmerman I, Kansen HM, van den Bos C, Zsiros J, van den Berg H, et al. Peripheral stem cell apheresis is feasible post 131Iodine-metaiodobenzylguanidine-therapy in high-risk neuroblastoma, but results in delayed platelet reconstitution. Clin Cancer Res. 2019;25:1012–21. https://doi.org/10.1158/1078-0432.CCR-18-1904.
Kiss F, Toth E, Miszti-Blasius K, Nemeth N. The effect of centrifugation at various g force levels on rheological properties of rat, dog, pig and human red blood cells. Clin Hemorheol Microcirc. 2016;62:215–27. https://doi.org/10.3233/CH-151965.
Coumans F, van Dalum G, Terstappen LW. CTC technologies and tools. Cytometry. 2018;93:1197–201. https://doi.org/10.1002/cyto.a.23684.
Tamminga M, Andree KC, Hiltermann TJN, Jayat M, Schuuring E, van den Bos H, et al. Detection of circulating tumor cells in the diagnostic leukapheresis product of non-small-cell lung cancer patients: comparing CellSearch® and ISET. Cancers. 2020;12:896 https://doi.org/10.3390/cancers12040896.
Zhang X, Wei L, Li J, Zheng J, Zhang S, Zou J. Epithelial-mesenchymal transition phenotype of circulating tumor cells is associated with distant metastasis in patients with NSCLC. Mol Med Rep. 2019;19:601–8. https://doi.org/10.3892/mmr.2018.9684.
Hosokawa M, Kenmotsu H, Koh Y, Yoshino T, Yoshikawa T, Naito T, et al. Size-based isolation of circulating tumor cells in lung cancer patients using a microcavity array system. PLoS ONE. 2013;8:e67466 https://doi.org/10.1371/journal.pone.0067466.
Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–29. https://doi.org/10.1038/nrclinonc.2017.44.
Mascalchi M, Falchini M, Maddau C, Salvianti F, Nistri M, Bertelli E, et al. Prevalence and number of circulating tumour cells and microemboli at diagnosis of advanced NSCLC. J Cancer Res Clin Oncol. 2015;142:195–200. https://doi.org/10.1007/s00432-015-2021-3.
Acknowledgements
During the study, R. Smith, J. Wheeler and J. Ladtkow (Terumo BCT, Lakewood Co, USA) provided advice and key insights into the apheresis procedure and technology. Procedures were run with the assistance of the personnel from Sanquin (Sanquin, Bloedvoorziening, Groningen, The Netherlands). We are very grateful for all of their expertise and efforts.
Funding
The authors are part of the CANCER-ID consortium, which has received support from the Innovative Medicines Initiative (IMI) Joint Undertaking under grant agreement no. 115749. Additional funding from the Dutch cancer funds (KWF subsidy no. 10491 from 2016) was received. Funding sources had no influence on data gathering, analysing, interpreting or the writing and publishing of the manuscript.
Author information
Authors and Affiliations
Contributions
Study concepts: LWMMT and HJMG. Study design: HJMG, MT, TJNH, KCA and ES. Data acquisition: MT and HJMG. Quality control of data and algorithms: MT, ES, HJMG and TJNH. Data analysis and interpretation: MT, TJNH and JMG. Statistical analysis: MT and HJMG. Manuscript preparation: MT. Manuscript editing: KCA, HvdB, TJNH, AM, DCJS, PL, WT, ES, LWMMT and HJMG. Manuscript review: PL, WT, ES, LWMMT and HJMG.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Medical Ethical Committee (NL55754.042.15) and was registered in the Dutch trial register (NL5423). Informed consent was obtained from all patients.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tamminga, M., Andree, K.C., van den Bos, H. et al. Leukapheresis increases circulating tumour cell yield in non-small cell lung cancer, counts related to tumour response and survival. Br J Cancer 126, 409–418 (2022). https://doi.org/10.1038/s41416-021-01634-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01634-0