Abstract
Background and aims
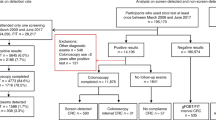
We aimed to evaluate the effects of switching to faecal immunochemical testing (FIT) on the cumulative 2-year incidence rate of interval cancers, interval cancer rate and test sensitivity within a mature population-based colorectal cancer screening programme consisting of six rounds of biennial guaiac faecal occult blood testing (gFOBT).
Methods
The FIT results were compared with those of gFOBT used in each of the previous two rounds. For the three rounds analysed, 279,041 tests were performed by 156,186 individuals. Logistic regression analysis was used to determine interval cancer risk factors (Poisson regression) and to compare the sensitivity of FIT to gFOBT.
Results
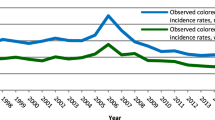
There were 612 cases of screen-detected cancers and 209 cases of interval cancers. The sex- and age-adjusted cumulative 2-year incidence rates of interval cancers were 55.7 (95% CI, 45.3–68.5), 42.4 (95% CI, 32.6–55.2) and 15.8 (95% CI, 10.9–22.8) per 100,000 person-years after the last two rounds of gFOBT and FIT, respectively. The FIT/gFOBT incidence rate ratio was 0.38 [95% CI, 0.27–0.54] (P < 0.001). Sex- and age-adjusted sensitivity was significantly higher with FIT than with gFOBT (OR = 6.70 [95% CI, 4.48–10.01], P < 0.0001).
Conclusions
This population-based study revealed a dramatic decrease in the cumulative incidence rates of interval cancers after switching from gFOBT to FIT. These data provide an additional incentive for countries still using gFOBT to switch to FIT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
The authors state that the data from their study are available on request from the Biostatistics Department of the University Hospital Center of Rennes (chloe.rousseau@chu-rennes.fr).
References
Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159:335–.e15.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Doubeni CA, Fedewa SA, Levin TR, Jensen CD, Saia C, Zebrowski AM, et al. Modifiable failures in the colorectal cancer screening process and their association with risk of death. Gastroenterology. 2019;156:63–74.e6.
Sanduleanu S, le Clercq CMC, Dekker E, Meijer GA, Rabeneck L, Rutter MD, et al. Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut. 2015;64:1257–67.
Moss S, Ancelle-Park R, Brenner H. European guidelines for quality assurance in colorectal cancer screening and diagnosis.—first edition faecal occult blood testing. Endoscopy. 2012;44:SE49–64.
Tinmouth J, Lansdorp-Vogelaar I, Allison JE. Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know. Gut. 2015;64:1327–37.
Robertson DJ, Lee JK, Boland RC, Dominitz JA, Giardiello FM, Johnson DA, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017;112:37–53.
Senore C, Basu P, Anttila A, Ponti A, Tomatis M, Vale DB, et al. Performance of colorectal cancer screening in the European Union Member States: data from the second European screening report. Gut. 2019;68:1232–44.
Wieten E, Schreuders EH, Grobbee EJ, Nieboer D, Bramer WM, Lansdorp-Vogelaar I, et al. Incidence of faecal occult blood test interval cancers in population-based colorectal cancer screening: a systematic review and meta-analysis. Gut. 2019;68:873–81.
Cardoso R, Guo F, Heisser T, Hoffmeister M, Brenner H. Utilisation of colorectal cancer screening tests in European countries by type of screening offer: results from the European Health Interview Survey. Cancers. 2020;12:1409.
Bretagne J-F, Piette C, Cosson M, Durand G, Lièvre A. Switching from guaiac to immunochemical faecal occult blood test increases participation and diagnostic yield of colorectal cancer screening. Dig Liver Dis. 2019;51:1461–9.
Piette C, Durand G, Bretagne J-F, Faivre J. Additional mailing phase for FIT after a medical offer phase: The best way to improve compliance with colorectal cancer screening in France. Dig Liver Dis. 2017;49:308–11.
Brierley JD, Gospodarowicz MK, Witterkind C (eds). The TNM classification of malignant tumors, 8th edn. New York, USA: Wiley; 2017.
Koïvogui A, Mab GL, Benamouzig R. Detection of colorectal neoplasia in a cohort before and after the change of faecal occult blood test in a french colorectal cancer screening program. Am J Gastroenterol. 2018;113:1891–9.
Vitellius C, Laly M, Banaszuk AS, Deherce I, Cornet N, Bertrais S, et al. Contribution of the OC Sensor® immunoassay in comparison to the Hemoccult II® guaiac-test in organized colorectal cancer screening. Eur J Epidemiol. 2019;34:163–72.
Moss S, Mathews C, Day TJ, Smith S, Seaman HE, Snowball J, et al. Increased uptake and improved outcomes of bowel cancer screening with a faecal immunochemical test: results from a pilot study within the national screening programme in England. Gut. 2017;66:1631–44.
Zorzi M, Hassan C, Senore C, Capodaglio G, Turrin A, Narne E, et al. Interval colorectal cancers after negative faecal immunochemical test in a 13-year screening programme. J Med Screen. 2021;28:131–9.
Guo F, De Brabander I, Francart J, Candeur M, Polus M, Van Eycken L, et al. Benefits of switching from guaiac-based faecal occult blood to faecal immunochemical testing: experience from the Wallonia–Brussels colorectal cancer screening programme. Br J Cancer. 2020;122:1109–17.
de Klerk CM, Vendrig LM, Bossuyt PM, Dekker E. Participant-related risk factors for false-positive and false-negative fecal immunochemical tests in colorectal cancer screening: systematic review and meta-analysis. Am J Gastroenterol. 2018;113:1778–87.
Wong MCS, Ching JYL, Chan VCW, Lam TY, Luk AK, Ng SS, et al. Factors associated with false-positive and false-negative fecal immunochemical test results for colorectal cancer screening. Gastrointest Endosc. 2015;81:596–607.
Stegeman I, de Wijkerslooth TR, Stoop EM, van Leerdam M, van Ballegooijen M, Kraaijenhagen RA, et al. Risk factors for false positive and for false negative test results in screening with fecal occult blood testing: Risk factors for false positive and false negative test. Int J Cancer. 2013;133:2408–14.
Cha JM, Suh M, Kwak MS, Sung NY, Choi KS, Park B, et al. Risk of interval cancer in fecal immunochemical test screening significantly higher during the summer months: results from the national cancer screening programme in Korea. Am J Gastroenterol. 2018;113:611–21.
Chausserie S, Levillain R, Puvinel J, Ferrand O, Ruiz A, Raginel T, et al. Seasonal variations do not affect the superiority of fecal immunochemical tests over guaiac tests for colorectal cancer screening: seasonal variation in FIT performance. Int J Cancer. 2015;136:1827–34.
Dancourt V, Hamza S, Manfredi S, Drouillard A, Bidan J-M, Faivre J, et al. Influence of sample return time and ambient temperature on the performance of an immunochemical faecal occult blood test with a new buffer for colorectal cancer screening. Eur J Cancer Prev. 2016;25:109–14.
Selby K, Levine EH, Doan C, Gies A, Brenner H, Quesenberry C, et al. Effect of sex, age, and positivity threshold on fecal immunochemical test accuracy: a systematic review and meta-analysis. Gastroenterology. 2019;157:1494–505.
Imperiale TF, Gruber RN, Stump TE, Emmett TW, Monahan PA. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: a systematic review and meta-analysis. Ann Intern Med. 2019;170:319–29.
Toes-Zoutendijk E, Kooyker AI, Dekker E, Spaander MCW, Opstal-van Winden, AWJ, Ramakers C, et al. Incidence of interval colorectal cancer after negative results from first-round fecal immunochemical screening tests, by cutoff value and participant sex and age. Clin Gastroenterol Hepatol. 2020;18:1493–1500.
Hirai HW, Tsoi KKF, Chan JYC, Wong SH, Ching JY, Wong MC, et al. Systematic review with meta-analysis: faecal occult blood tests show lower colorectal cancer detection rates in the proximal colon in colonoscopy-verified diagnostic studies. Aliment Pharm Ther. 2016;43:755–64.
Lu M, Luo X, Li N, Chen H, Dai M. Diagnostic accuracy of fecal occult blood tests for detecting proximal versus distal colorectal neoplasia: a systematic review and meta-analysis. Clin Epidemiol. 2019;11:943–54.
Steele RJ, McClements P, Watling C, Libby G, Weller D, Brewster DH, et al. Interval cancers in a FOBT-based colorectal cancer population screening programme: implications for stage, gender and tumour site. Gut. 2012;61:576–81.
van der Vlugt M, Grobbee EJ, Bossuyt PMM, Bos A, Bongers E, Spijker W, et al. Interval colorectal cancer incidence among subjects undergoing multiple rounds of fecal immunochemical testing. Gastroenterology. 2017;153:439–.e2.
van de Veerdonk W, Hoeck S, Peeters M, Van Hal G, Francart J, De Brabander I. Occurrence and characteristics of faecal immunochemical screen‐detected cancers vs non–screen‐detected cancers: results from a Flemish colorectal cancer screening programme. United Eur Gastroenterol J. 2020;8:185–94.
Vicentini M, Zorzi M, Bovo E, Mancuso P, Zappa M, Manneschi G, et al. Impact of screening programme using the fecal immunochemical test on stage of colorectal cancer: results from the IMPATTO study. Int J Cancer. 2019;145:110–21.
Giorgi Rossi P, Carretta E, Mangone L, Baracco S, Serraino D, Zorzi M. Incidence of interval cancers in faecal immunochemical test colorectal screening programmes in Italy. J Med Screen. 2018;25:32–39.
Mancini S, Bucchi L, Giuliani O, Ravaioli A, Vattiato R, Baldacchini F, et al. Proportional incidence of interval colorectal cancer in a large population-based faecal immunochemical test screening programme. Dig Liver Dis. 2020;52:452–6.
Bordet M, Bretagne J-F, Piette C, Rousseau C, Grainville T, Cosson M, et al. Reappraisal of the characteristics, management, and prognosis of intramucosal colorectal cancers and their comparison with T1 carcinomas. Gastrointest Endosc. 2021;93:477–85.
Acknowledgements
The authors acknowledge the contributions of the general practitioners, gastroenterologists and surgeons in the district of Ille-et-Vilaine. They also acknowledge the staff in charge of the database at the screening centre, particularly Samuel Foucrit, a computer scientist.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Conception and design (JFB, CP, MC and AL); analysis and interpretation of the data (CR, JFB, AL, AC, CP and MC) and drafting of the article (JFB and AL); all of the authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The colorectal cancer screening programme was declared and approved by the CNIL (Commission Nationale de l’Informatique et des Libertés) on August 30, 2002 (no. 812571). This research was approved by the CCTIRS (Comité Consultatif pour le Traitement de l’Information en Matière de Recherche dans le Domaine de la Santé).
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bretagne, JF., Carlo, A., Piette, C. et al. Significant decrease in interval colorectal cancer incidence after implementing immunochemical testing in a multiple-round guaiac-based screening programme. Br J Cancer 125, 1494–1502 (2021). https://doi.org/10.1038/s41416-021-01546-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01546-z