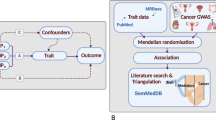
Abstract
Background
Cortisol’s immunosuppressive, obesogenic, and hyperglycaemic effects suggest that it may play a role in cancer development. However, whether cortisol increases cancer risk is not known. We investigated the potential causal association between plasma cortisol and risk of overall and common site-specific cancers using Mendelian randomisation.
Methods
Three genetic variants associated with morning plasma cortisol levels at the genome-wide significance level (P < 5 × 10−8) in the Cortisol Network consortium were used as genetic instruments. Summary-level genome-wide association study data for the cancer outcomes were obtained from large-scale cancer consortia, the UK Biobank, and the FinnGen consortium. Two-sample Mendelian randomisation analyses were performed using the fixed-effects inverse-variance weighted method. Estimates across data sources were combined using meta-analysis.
Results
A standard deviation increase in genetically predicted plasma cortisol was associated with increased risk of endometrial cancer (odds ratio 1.50, 95% confidence interval 1.13–1.99; P = 0.005). There was no significant association between genetically predicted plasma cortisol and risk of other common site-specific cancers, including breast, ovarian, prostate, colorectal, lung, or malignant skin cancer, or overall cancer.
Conclusions
These results indicate that elevated plasma cortisol levels may increase the risk of endometrial cancer but not other cancers. The mechanism by which this occurs remains to be investigated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data used in this study are publicly available summary-level data, with the relevant studies cited.
References
Katsu Y, Iguchi T. Cortisol. In: Takei Y, Ando H & Tsutsui K, editors. Handbook of hormones. Oxford, UK: Academic Press; 2016.
Antonova L, Aronson K, Mueller CR. Stress and breast cancer: from epidemiology to molecular biology. Breast Cancer Res. 2011;13:208.
Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13.
Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–35.
van der Valk ES, Savas M, van Rossum EFC. Stress and obesity: are there more susceptible individuals? Curr Obes Rep. 2018;7:193–203.
Nead KT, Sharp SJ, Thompson DJ, Painter JN, Savage DB, Semple RK, et al. Evidence of a causal association between insulinemia and endometrial cancer: a Mendelian randomization analysis. J Natl Cancer Inst. 2015;107:djv178.
Kruk J, Aboul-Enein BH, Bernstein J, Gronostaj M. Psychological stress and cellular aging in cancer: a meta-analysis. Oxid Med Cell Longev. 2019;2019:1270397.
Shin KJ, Lee YJ, Yang YR, Park S, Suh PG, Follo MY, et al. Molecular mechanisms underlying psychological stress and cancer. Curr Pharm Des. 2016;22:2389–402.
Yang T, Qiao Y, Xiang S, Li W, Gan Y, Chen Y. Work stress and the risk of cancer: a meta-analysis of observational studies. Int J Cancer. 2019;144:2390–400.
Bahri N, Fathi Najafi T, Homaei Shandiz F, Tohidinik HR, Khajavi A. The relation between stressful life events and breast cancer: a systematic review and meta-analysis of cohort studies. Breast Cancer Res Treat. 2019;176:53–61.
Bolton JL, Hayward C, Direk N, Lewis JG, Hammond GL, Hill LA, et al. Genome wide association identifies common variants at the SERPINA6/SERPINA1 locus influencing plasma cortisol and corticosteroid binding globulin. PLoS Genet. 2014;10:e1004474.
Broad Institute of MIT and Harvard. The Genotype-Tissue Expression (GTEx) portal. 2021. https://gtexportal.org/home/. Accessed 12 Feb 2021.
Michailidou K, Lindstrom S, Dennis J, Beesley J, Hui S, Kar S, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92–4.
O’Mara TA, Glubb DM, Amant F, Annibali D, Ashton K, Attia J, et al. Identification of nine new susceptibility loci for endometrial cancer. Nat Commun. 2018;9:3166.
Phelan CM, Kuchenbaecker KB, Tyrer JP, Kar SP, Lawrenson K, Winham SJ, et al. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat Genet. 2017;49:680–91.
Schumacher FR, Al Olama AA, Berndt SI, Benlloch S, Ahmed M, Saunders EJ, et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018;50:928–36.
Wang Y, McKay JD, Rafnar T, Wang Z, Timofeeva MN, Broderick P, et al. Rare variants of large effect in BRCA2 and CHEK2 affect risk of lung cancer. Nat Genet. 2014;46:736–41.
FinnGen consortium. FinnGen Documentation of R4 release, 2020. https://finngen.gitbook.io/documentation/. Accessed 10 Dec 2020.
Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–34.
Astle WJ, Elding H, Jiang T, Allen D, Ruklisa D, Mann AL, et al. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell. 2016;167:1415.e19–29.e19.
Marchini J, Howie B. Genotype imputation for genome-wide association studies. Nat Rev Genet. 2010;11:499–511.
Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42:1497–501.
Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46:1734–9.
Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. 2019;28:166–74.
Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–16.
Neale Laboratory. GWAS round 2 of the UK Biobank. Results shared 1st August 2018. 2018. http://www.nealelab.is/uk-biobank. Accessed 5 May 2021.
Rodriguez AC, Blanchard Z, Maurer KA, Gertz J. Estrogen signaling in endometrial cancer: a key oncogenic pathway with several open questions. Horm Cancer. 2019;10:51–63.
Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26:252–8.
Dupuis CC, Storr HL, Perry LA, Ho JT, Ahmed L, Ong KK, et al. Abnormal puberty in paediatric Cushing’s disease: relationship with adrenal androgen, sex hormone binding globulin and gonadotrophin concentrations. Clin Endocrinol. 2007;66:838–43.
Woods NF, Mitchell ES, Smith-Dijulio K. Cortisol levels during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2009;16:708–18.
Grant SD, Pavlatos FC, Forsham PH. Effects of estrogen therapy on cortisol metabolism. J Clin Endocrinol Metab. 1965;25:1057–66.
Edwards KM, Mills PJ. Effects of estrogen versus estrogen and progesterone on cortisol and interleukin-6. Maturitas. 2008;61:330–3.
Vahrenkamp JM, Yang C-H, Rodriguez AC, Jarboe EA, Janat-Amsbury MM. Clinical and genomic crosstalk between glucocorticoid receptor and estrogen receptor α in endometrial cancer. Cell Rep. 2018;22:P2995–3005.
Byrne, FL, Martin, AR, Kosasih, M, Caruana, BT, Farrell, R. The role of hyperglycemia in endometrial cancer pathogenesis. Cancers. 2020;12:1191.
Acknowledgements
The authors would like to thank the investigators of the Breast Cancer Association Consortium (BCAC), Endometrial Cancer Association Consortium (ECAC), Genetic Investigation of ANthropometric Traits (GIANT) consortium, International Lung Cancer Consortium (ILCCO), Meta-Analyses of Glucose and Insulin-related traits consortium (MAGIC), Ovarian Cancer Association Consortium (OCAC), Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL) consortium, and the FinnGen consortium for sharing summary-level GWAS data. Analyses of UK Biobank data were performed under application 29202.
Funding
SCL acknowledges research support from the Swedish Research Council (Vetenskapsrådet, 2016-01042 and 2019-00977), the Swedish Research Council for Health, Working Life and Welfare (Forte, 2018-00123), and the Swedish Heart-Lung Foundation (Hjärt-Lungfonden, 20190247). SK is supported by United Kingdom Research and Innovation Future Leaders Fellowship (MR/T043202/1). SB is supported by Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (204623/Z/16/Z). During the conduction of this study, EA was supported by the EU/EFPIA Innovative Medicines Initiative Joint Undertaking BigData@Heart grant no. 116074 and is currently funded by the British Heart Foundation Programme Grant RG/18/13/33946. This work was supported by core funding from: the UK Medical Research Council (MR/L003120/1), the British Heart Foundation (RG/13/13/30194; RG/18/13/33946), and the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014)*. This work was also supported by Health Data Research UK, which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation, and Wellcome. *The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
SCL had full access to the data. SCL, SB, and EA designed the study. SCL performed the statistical analyses and created the figure. SCL, W-HL, and EA drafted the manuscript. SCL, W-HL, SK, SB, and EA interpreted the data and edited the manuscript. All authors have given final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Ethics approval and consent to participate had been obtained. The present analyses were approved by the Swedish Ethical Review Authority. The study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Larsson, S.C., Lee, WH., Kar, S. et al. Assessing the role of cortisol in cancer: a wide-ranged Mendelian randomisation study. Br J Cancer 125, 1025–1029 (2021). https://doi.org/10.1038/s41416-021-01505-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01505-8
This article is cited by
-
Links between the genetic determinants of morning plasma cortisol and body shape: a two-sample Mendelian randomisation study
Scientific Reports (2024)
-
Identification of predictive biomarkers for endometrial cancer diagnosis and treatment response monitoring using plasma metabolome profiling
Cancer & Metabolism (2023)