Abstract
Background
Pazopanib is active in refractory soft-tissue sarcoma (STS) and significantly prolongs PFS. Prior studies of combinations of metronomic topotecan with pazopanib have indicated preclinical evidence of response in patients with sarcoma.
Methods
This prospective, single arm, phase II study evaluated the efficacy of the combination of pazopanib with topotecan in patients with metastatic or unresectable non-adipocytic STS. Furthermore, it incorporated exploratory arms for osteosarcoma and liposarcoma. The primary endpoint was progression-free rate at 12 weeks in the non-adipocytic STS cohort.
Results
57.5% of patients in the non-adipocytic STS cohort were progression free at 12 weeks, which did not meet the primary endpoint of the study (66%). The exploratory osteosarcoma cohort exceeded previously established phase II trial comparator data benchmark of 12% with a PFR at 12 weeks of 69.55%. Treatment with the combination of pazopanib and topotecan was accompanied by a grade 3 or 4 toxicities in most patients.
Conclusions
In this prospective trial in refractory metastatic or unresectable STS and osteosarcoma, the combination of pazopanib with topotecan did not meet its primary endpoint of progression-free rate at 12 weeks. The combination of pazopanib with topotecan was associated with a high degree of toxicity.
Similar content being viewed by others
Background
Soft-tissue sarcomas (STS) are a rare family of cancers that originate from mesenchymal tissue.1 Combined, all subtypes of sarcoma account for ~1% of adult cancers.2 In 2020, the projected annual incidence of sarcoma in the United States will be 16,730, with 6070 deaths occurring secondary to the disease. Historically, first-line therapy for most varieties of metastatic STS has involved treatment with systemic chemotherapy involving an anthracycline—either alone or in combination.3 In the front line EORTC 62012 study, doxorubicin was compared to doxorubicin with ifosfamide, demonstrating nonsignificant differences in overall survival (OS) between groups.4 These results are in line with other studies, and historical analyses that have placed the 2-year survival for advanced STS at 22%.5 While targeted therapies, such as imatinib for GIST, have revolutionised treatment for some sarcoma subtypes, subsequent line therapy for most STS has been unable to achieve such durable responses.6,7 In light of the aforementioned, there is a clear need for further development, and study of later line therapies for patients with STS.
Angiogenesis is an established mechanism of tumour growth in malignancy, including STS, with metastatic potential associated with degree of vascularisation.8 Although a complicated process, angiogenesis is in large part driven by the presence of VEGF. The VEGF pathway may be inhibited by tyrosine kinase inhibitors (TKIs), such as pazopanib.9 In 2012, the FDA approved the TKI, pazopanib, in advanced and metastatic non-adipocytic STS previously treated with chemotherapy based on the phase III randomised, double-blind, placebo-controlled PALETTE study.10 In this trial, median progression-free survival (PFS) was significantly improved at 4.6 months versus 1.6 months in the pazopanib and placebo groups, respectively (HR 0.31, 95% CI 0.24–0.4). There remained some uncertainties, however, regarding the role of pazopanib in the care of liposarcoma, with recent studies indicating perhaps some efficacy alone, or in combination with cytotoxic treatment.11,12
Topotecan, when used as treatment for STS demonstrated tolerability, as well as objective response in leiomyosarcoma.13,14 Combination regimens with topotecan likewise have shown efficacy in populations of patients with relapsed and refractory osteosarcoma.15 Preclinical data involving combination therapy of both metronomic topotecan, and pazopanib may enhance anti-tumour and anti-angiogenic effects.16,17 A phase I study of pazopanib and topotecan was conducted in 67 patients with advanced solid tumours and reported in 2015.18 This trial included patients with STS and osteosarcoma, with results showing a recommended dose for topotecan of 8 mg weekly in conjunction with 800 mg daily of pazopanib. Given the relative safety of the regimen and preclinical evidence of response, we sought to better qualify the effectiveness of dual therapy with pazopanib and topotecan in patients with metastatic and non-resectable STS and osteosarcoma.
Methods
Patient eligibility
Eligible patients for this study had a diagnosis of recurrent or metastatic, non-resectable soft-tissue sarcoma that had failed at least one prior therapy, or metastatic or unresectable osteosarcoma. Furthermore, patients were required to be 18 years or older, have ECOG performance status 0–1, and have measurable disease by RECIST 1.1 within 4 weeks prior to registration. Patients with alveolar soft-part sarcoma, Ewing sarcoma, and GIST were excluded. Additionally, this trial excluded patients who had previously received pazopanib or topotecan.
Clinical trial design and conduct
The STS, osteosarcoma and liposarcoma cohorts of this study were part of a multicenter, phase II, non-randomised, non-comparative trial that recruited patients from six centers across the United States. The primary endpoint of this study was progression-free rate (PFR) at 12 weeks. This study was approved by institutional review boards at all participating sites prior to initiation. All patients provided written informed consent prior to participation.
Treatment
Enrolled patients received pazopanib at a fixed starting dose of 800 mg daily, in combination with oral topotecan 8 mg on days 1, 8 and 15 of each cycle. Total cycle duration was 28 days. Treatment was continued until disease progression as assessed by RECIST v1.1, or patient or physician-initiated discontinuation.
Dose modification for toxicities
Toxicities were evaluated using criterion from common terminology criteria for adverse events (CTCAE) version 4.03. Assessment of toxicity occurred after cycle 1 day 1 of treatment every other week for 8 weeks, and then with administration of each subsequent cycle. For topotecan, three dose reductions were permitted based on side effects experienced, with a minimum dose of 2 mg on days 1, 8 and 15. When appropriate, pazopanib was held up to 3 weeks until resolution of toxicities, but also was allowed three dose reductions to a minimum of 200 mg daily.
Response assessment
Tumour assessments were performed utilising RECIST version 1.1. Patients were re-evaluated for response 6 weeks and 12 weeks after treatment initiation. Following assessment at 12 weeks, patients were imaged every 2 cycles (every 8 weeks). A 7-day window was allowed for each scan.
Statistical analysis
This Phase II trial utilised a Simon two-stage design. Patients with metastatic disease were assigned to one of three cohorts by histologic diagnosis. Cohort 1 was patients with non-liposarcoma STS, Cohort 2 incorporated patients with osteosarcoma and Cohort 3 was composed of patients with de-differentiated or high grade liposarcoma. The study was powered to detect a 20% improvement in the historic PD-free rate of 55% for single agent pazopanib at 12 weeks (or an 11% absolute improvement) in cohort 1, while cohorts 2 and 3 were exploratory. An increased PFR at 12 weeks was deemed clinically meaningful, with a change in PFR of less than 11% considered ineffective. A total of 92 evaluable patients was required to detect this difference with an 80% power and an alpha level of 10%. Exploratory 20 patient liposarcoma and 36 patient osteosarcoma arms were enrolled for assessment of feasibility.
Secondary endpoints of overall response rate (CR + PR), clinical benefit rate (CR + PR + SD), overall survival (OS) and progression-free survival (PFS) based on incidence of CTAE v 4.03 events were also assessed.
Overall response rate and clinical benefit rate are given in percentages with 95% confidence intervals estimated based on assumptions of the binomial distribution. A one sample Chi-squared test was employed to test the null hypothesis that these proportions equal 50%. OS and PFS rates, their 95% confidence intervals, median OS and PFS times were estimated via the Kaplan–Meier method and differences between cohorts was assessed via the log-rank test. Analyses were conducted in R 3.6.3.
Results
Patient characteristics
Between March 2015 and May 2020, 106 patients were enrolled into cohort 1 (non-adipocytic STS), 28 patients were enrolled into cohort 2 (osteosarcoma) and 19 patients were enrolled into cohort 3 (liposarcoma). Of 106 patients enrolled in cohort 1, 105 were evaluable for efficacy, and 106 were evaluable for toxicity. Within the three cohorts, demographics were comparable to those of other studies of patients with metastatic sarcoma. In cohort 1, 49.1% of patients had leiomyosarcoma, 9.4% had synovial sarcoma and 41.5% being of other subtypes (Table 1). Notably, no patients had the diagnosis of solitary fibrous tumour. In cohort 3, 74% had de-differentiated, 10% had pleomorphic and 16% had myxoid/round cell histologies. Median age of cohort 1 was 57 years (range: 24–80); median age of cohort 2 was 38 years (range: 18–72) and median age of cohort 3 was 61 years (range: 32–77). While 63.2% of patients in cohort 1 were female, 57.1% and 68.4% were male in cohorts 2 and 3, respectively. This discrepancy is largely explained by the high percentage of uterine leiomyosarcoma in cohort 1. Most patients in both cohorts were of ECOG status 1. 79% of patients in cohort 1, 75% of patients in cohort 2 and 63.2% of patients in cohort 3 had received at least three prior lines of therapy for their disease. The data cut-off date was May 2020. Median follow-up time for cohorts 1, 2 and 3 were 35.8, 25.2 and 28.8 months, respectively.
Efficacy
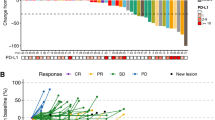
Within the first stage, 32 of 54 (59.2%) evaluable patients in cohort 1 were alive and progression free at 12 weeks, exceeding prespecified thresholds and triggering full accrual to all cohorts. Data for the 105 evaluable patients in cohort 1, stages 1 and 2 combined, 22 evaluable patients in cohort 2 and 18 evaluable patients in cohort 3 is presented (Fig. 1). Between the two stages of this Simon optimum phase II study, 57.5% of patients in cohort 1 were progression free at 12 weeks compared to 69.5% of patients in cohort 2 and 31.2% of patients in cohort 3. At 24 weeks, PFR was 39% (95% CI 30.1–50.5%), 45.4% (95% CI 28.7–71.8%) and 22.2% (95% CI 9.3–52.7%) for cohorts 1, 2 and 3, respectively. Median PFS for cohort 1 was 4.3 months, 4.5 months in cohort 2 and 1.4 months in cohort 3. CR and PR rates were 1% and 8%, for cohort 1, respectively, while 1 PR was achieved in cohort 2, and no CRs or PRs were achieved in cohort 3. At the time of the data cut-off, 20% of patients were progression free at 12 months in cohort 1, 18.2% of patients were progression free at 12 months in cohort 2 and 14.81% of patients were progression free in cohort 3. These data did not meet the primary endpoint of the study, indicating a lack of efficacy of the therapy in cohort 1 at the 12 week time point.
Data regarding overall survival are viewable in Fig. 2. Median overall survival for patients in cohort 1 was 10.9 months. In cohort 2, median overall survival was 11.1 months (95% CI 7.0–20.37), while overall survival was 12.8 months in cohort 3 (95% CI 10.61–Not reached). No patients were receiving treatment at the time of study closure.
Overall response rate within cohort 1 was 7.5%, while that of cohorts 2 and 3 were 4%, and 0%, respectively. Clinical benefit rate (CBR) was 71% (95% CI 61–79%), 79% (95% CI 59–90%) and 44% (95% CI 24–66%), for cohorts 1, 2 and 3, respectively.
Toxicity
Treatment-related AEs were beyond those previously seen in prior studies of pazopanib and topotecan. Table 2 lists the most common AEs deemed likely related to study medications occurring in at least three patients on study. Side effects were comparable across cohorts, as each followed the same treatment protocol.
Most common AEs were anaemia, thrombocytopenia and neutropenia, which were experienced by most patients on study. Anaemia was seen in 98% of patients, while thrombocytopenia and neutropenia were seen in 88% and 79% of patients, respectively. Among these, 20% and 1%, respectively, of anaemias were grade 3 or 4. Thrombocytopenic events were grade 3 or 4 at rates of 30% and 11%, respectively. Forty six percent of patients experienced grade 3 and 7% experienced grade 4 neutropenia. Hypertension was present in the study population at a rate of 23% for grade 3 or higher. Hyponatremia was also present at a rate of 11% for grade 3. In total, there were three treatment-related deaths. These were bladder perforation, thromboembolic event and colonic perforation. These adverse events had not been reported in the prior phase I study of pazopanib combined with topotecan.
Discontinuation rate due to toxicities were 29% and 38% required dose interruption and reduction due to adverse events.
Discussion
At the time of writing, this study represented the first multicenter, open-label phase 2 trial of pazopanib in combination with cytotoxic therapy for patients with previously treated metastatic, and unresectable soft-tissue sarcoma and osteosarcoma. The objective of this study was to determine the role of the combination therapy in patients with these disease states. Across all cohorts, patients were heavily pretreated, with 79%, 75% and 63.2% of patients having received 3 more lines of therapy in cohorts 1, 2 and 3, respectively, (Table 1).
Sixty-three of 105 (60%) patients enrolled in cohort 1 were progression free at 12 weeks, which was unfortunately below the 66% threshold required for statistical evidence of efficacy. These results did indicate a numerical improvement in PFR at 12 weeks over that of pazopanib alone in previous studies, 60% versus 55%, respectively.10 Prior studies of cytotoxic, and tyrosine kinase inhibition alone have generally yielded median PFS of 2–4 months after first-line therapy.10,19 Twenty percent of patients in cohort 1, 18% of patients in cohort 2 and 14.8% of patients in cohort 3 were alive and progression free at 1 year, indicating perhaps some durability of study agent effect. Interestingly, one complete response was achieved within cohort 1, as well as eight partial responses. In analyzing PFR by histologic subtype, 23.9% of the 46 patients with leiomyosarcoma were progression free at 1 year, which was numerically highest among the subgroups. Among other non-adipocytic STS subtypes, PFR at 1 year was 19.4%, while those of liposarcoma and osteosarcoma were 14.8% and 22.3%, respectively. In this trial, the median overall survival for cohort 1 was 10.9 months, 11.11 months in cohort 2 and 12.81 months in cohort 3. This is in line with prior studies of patients treated with chemotherapy, or TKI agents with median OS between 11 and 13 months.7,10 Median OS was longest in the subpopulation with liposarcoma.
Although the combination of pazopanib and topotecan did not meet predefined endpoints, other recent trials of the combination of gemcitabine with pazopanib have demonstrated efficacy above that of single agent pazopanib alone.12 In the PAPAGEMO study, patients with anthracycline and/or ifosfamide refractory STS had a PFS of 5.6 months with gemcitabine and pazopanib, as opposed to 2.0 months with single agent pazopanib (HR 0.58, 95% CI 0.36–0.92). Median prior lines of therapy was 2 in the PAPAGEMO trial, with 30% of patients having received more than 2. Given 79% of patients in our trial had received at least three prior lines of therapy, these populations may not be directly comparable.
The phase II comparator trial data published in 2016 indicated an event-free survival rate, for patients with recurrent osteosarcoma enrolled in seven phase II trials through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group of 12% at 4 months.20 Utilising this efficacy benchmark, a threshold of 11 of 36 potentially enrolled patients with PFS greater than 20 weeks was needed in order to demonstrate efficacy. In our study, this level was exceeded with PFR of 45.5% at 6 months, indicating a high likelihood of efficacy in the treatment of this disease or an effect from pazopanib alone.
While our combination therapy exceeds this threshold, one must nonetheless consider combination of pazopanib and topotecan within the context of other recent studies for relapsed and refractory osteosarcoma.21,22 It is also important to note that the population contained in our exploratory cohort 2 differs from previously described demographics for osteosarcoma. Data from the EURAMOS-1 trial indicates median age at diagnosis of 14 years, while cohort 2 had a median age of 38.23 Mechanisms of pathogenesis, and tolerance of therapeutic agents may differ significantly between age groups, and, as such, consideration of a randomised, phase 2 study incorporating pediatric patients may be warranted.24 Furthermore, prior early phase studies in a pediatric population utilising combinations of cytotoxic medications and TKIs indicate tolerance of combinations.25
The AEs associated with this trial were higher than the previously conducted Phase I study.18 Most common grade 3 and 4 toxicities were hypertension and hematologic toxicity, which are well known effects of pazopanib and topotecan, respectively. Higher grade hypertension was managed with resolution of the AE in most cases. The discontinuation rate was higher than noted in prior studies at 29%, including recent data from the randomised phase II PAPAGEMO trial.12
In conclusion, despite early phase and preclinical promise, the combination of pazopanib and topotecan did not meet its primary endpoint of progression-free rate greater than or equal to 66% at 12 weeks in non-adipocytic STS and liposarcoma cohorts.20 Based on these results, although in line with historical comparisons, we do not recommend initiation of a phase III study of this combination of therapy in these patient populations.
References
Clark, M. A., Fisher, C., Judson, I. & Meirion Thomas, J. Soft-tissue sarcomas in adults. N. Engl. J. Med. 353, 701–711 (2005).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020).
Benjamin, R. S. & George, S. Soft Tissue Sarcoma Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl Compr. Canc. Netw. 16, 536–563 (2018).
Judson, I., Verweij, J., Gelderblom, H., Hartmann, J. T., Schöffski, P., Blay, J. Y. et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 15, 415–423 (2014).
Van Glabbeke, M., van Oosterom, A. T., Oosterhuis, J. W., Mouridsen, H., Crowther, D., Somers, R. et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens–a European Organization for Research and Treatment of Cancer Soft Tissue and Bone. J. Clin. Oncol. 17, 150–157 (1999).
Verweij, J., Casali, P. G., Zalcberg, J., LeCesne, A., Reichardt, P., Blay, J.-Y. et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364, 1127–1134 (2004).
Demetri, G. D., Von Mehren, M., Jones, R. L., Hensley, M. L., Schuetze, S. M., Staddon, A. et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J. Clin. Oncol. 34, 786–793 (2016).
Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 29, asonc02906q0015 (2002).
Ferrara, N., Gerber, H.-P. & LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 9, 669–676 (2003).
Van Der Graaf, W. T. A., Blay, J. Y., Chawla, S. P., Kim, D. W., Bui-Nguyen, B., Casali, P. G. et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379, 1879–1886 (2012).
Grünwald, V., Karch, A., Schuler, M., Schöffski, P., Kopp, H.-G., Bauer, S. et al. Randomized comparison of pazopanib and doxorubicin as first-line treatment in patients with metastatic soft tissue sarcoma age 60 years or older: results of a German Intergroup Study. J. Clin. Oncol. 38, 3555–3564 (2020).
Schmoll, H.-J., Lindner, L. H., Reichardt, P., Heißner, K., Kopp, H.-G., Kessler, T. et al. Efficacy of pazopanib with or without gemcitabine in patients with anthracycline- and/or ifosfamide-refractory soft tissue sarcoma. JAMA Oncol. 7, 255 (2021).
Miller, D. S., Blessing, J. A., Kilgore, L. C., Mannel, R. & Van Le, L. Phase II trial of topotecan in patients with advanced, persistent, or recurrent uterine leiomyosarcomas. Am. J. Clin. Oncol. 23, 355–357 (2000).
Reichardt, P., Oechsle, K., Pink, D., Bokemeyer, C., Schneller, F., Issels, R. et al. An open label, non-comparative phase II study of topotecan as salvage treatment for patients with soft tissue sarcoma. Invest. New Drugs 21, 481–486 (2003).
Saylors, R. L., Stine, K. C., Sullivan, J., Kepner, J. L., Wall, D. A., Bernstein, M. L. et al. Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: a Pediatric Oncology Group Phase II Study. J. Clin. Oncol. 19, 3463–3469 (2001).
Kumar, S., Mokhtari, R. B., Sheikh, R., Wu, B., Zhang, L., Xu, P. et al. Metronomic oral topotecan with pazopanib is an active antiangiogenic regimen in mouse models of aggressive pediatric solid tumor. Clin. Cancer Res. 17, 5656–5667 (2011).
Hashimoto, K., Man, S., Xu, P., Cruz-Munoz, W., Tang, T., Kumar, R. et al. Potent preclinical impact of metronomic low-dose oral topotecan combined with the antiangiogenic drug pazopanib for the treatment of ovarian cancer. Mol. Cancer Ther. 9, 996–1006 (2010).
Kerklaan, B. M., Lolkema, M. P. J., Devriese, L. A., Voest, E. E., Nol-Boekel, A., Mergui-Roelvink, M. et al. Phase I and pharmacological study of pazopanib in combination with oral topotecan in patients with advanced solid tumours. Br. J. Cancer 113, 706–715 (2015).
Schöffski, P., Chawla, S., Maki, R. G., Italiano, A., Gelderblom, H., Choy, E. et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 387, 1629–1637 (2016).
Lagmay, J. P., Krailo, M. D., Dang, H., Kim, A., Hawkins, D. S., Beaty, O. et al. Outcome of patients with recurrent osteosarcoma enrolled in seven Phase II trials through children’s cancer group, Pediatric Oncology Group, and Children’s Oncology Group: Learning From the Past to Move Forward. J. Clin. Oncol. 34, 3031–3038 (2016).
Duffaud, F., Mir, O., Boudou-Rouquette, P., Piperno-Neumann, S., Penel, N., Bompas, E. et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 20, 120–133 (2019).
Italiano, A., Mir, O., Mathoulin-Pelissier, S., Penel, N., Piperno-Neumann, S., Bompas, E. et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 21, 446–455 (2020).
Smeland, S., Bielack, S. S., Whelan, J., Bernstein, M., Hogendoorn, P., Krailo, M. D. et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur. J. Cancer 109, 36–50 (2019).
Mirabello, L., Troisi, R. J. & Savage, S. A. Osteosarcoma incidence and survival rates from 1973 to 2004. Cancer 115, 1531–1543 (2009).
Gaspar, N., Sirvent, F. J. B., Venkatramani, R., Longhi, A., Lervat, C., Casanova, M. et al. Phase I combination dose-finding/phase II expansion cohorts of lenvatinib + etoposide + ifosfamide in patients (pts) aged 2 to ≤ 25 years with relapsed/refractory (r/r) osteosarcoma. Ann. Oncol. 30, v688 (2019).
Acknowledgements
We are indebted to all the patients, and co-investigators who have participated in this trial. We want to thank the following collaborators of their assistance in preparation, and funding of this project.
Author information
Authors and Affiliations
Contributions
Manuscript preparation: B.S., M.A. Study design: N.M., M.A. Patient data and study materials: M.M., S.A., S.R., V.M., A.H., P.O., J.C., S.A., R.C., S.O., B.V.T., M.A. Data analysis and interpretation: B.S., M.A., I.H. Manuscript editing and review: All.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board of each participating institution—Northwestern University, University of Iowa, Mayo Clinic, Medical College of Wisconsin and Washington University School of Medicine. Furthermore, written consent was obtained from all participants.
Consent to publish
Not applicable.
Data availability
All data are available upon request.
Competing interests
The authors declare no competing interests.
Funding information
Research was sponsored by Northwestern University, with limited collaborative support from Novartis Pharmaceuticals.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schulte, B., Mohindra, N., Milhem, M. et al. Phase II study of pazopanib with oral topotecan in patients with metastatic and non-resectable soft tissue and bone sarcomas. Br J Cancer 125, 528–533 (2021). https://doi.org/10.1038/s41416-021-01448-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01448-0