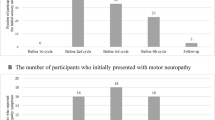
Abstract
Background
The prevalence of persistent peripheral neuropathy (PN) in early-stage breast cancer (ESBC) survivors is largely unknown. We explored the occurrence and risk factors of PN among long-term ESBC survivors treated with taxane chemotherapy.
Methods
A population-based cohort of 884 recurrence-free ESBC survivors diagnosed 2010–2015 in the South East Health Care region, Sweden and 1768 control women without prior cancer received a postal questionnaire that included the European Organisation for Research and Treatment of Cancer chemotherapy-induced peripheral neuropathy (CIPN20) items. Prevalence, relative risks (RRs) (Poisson regression) and risk factors (binomial regression) were calculated. Adjustments were made for confounding factors (e.g. age, body mass index, comorbidities).
Results
The response rate was 79% for survivors and 59% for controls. The median time post taxane was 3.6 years (1.5–7.3 years). The adjusted RR was highest (RR 1.8) for “tingling/numbness of toes/feet”. Individual sensory symptoms occurred in 8.9–48.4% and motor symptoms in 7.2–61.3% of survivors; the most prevalent symptoms were “difficulty opening jar” and “cramps in feet”. Paclitaxel, older age, overweight, diabetes mellitus, vibrating hand tools, autoimmune disease and smoking were independent risk factors.
Conclusions
PN was more common among ESBC survivors than control women and many symptoms persisted over time. Risk factors should be considered when treatment decisions are made.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Peto, R., Davies, C., Godwin, J., Gray, R., Pan, H. C., Clarke, M. et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379, 432–444 (2012).
Bedard, P. L., Di Leo, A. & Piccart-Gebhart, M. J. Taxanes: optimizing adjuvant chemotherapy for early-stage breast cancer. Nat. Rev. Clin. Oncol. 7, 22–36 (2010).
Mols, F., Beijers, T., Vreugdenhil, G. & van de Poll-Franse, L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support. Care Cancer 22, 2261–2269 (2014).
Eckhoff, L., Knoop, A., Jensen, M. B. & Ewertz, M. Persistence of docetaxel-induced neuropathy and impact on quality of life among breast cancer survivors. Eur. J. Cancer 51, 292–300 (2015).
Tanabe, Y., Hashimoto, K., Shimizu, C., Hirakawa, A., Harano, K., Yunokawa, M. et al. Paclitaxel-induced peripheral neuropathy in patients receiving adjuvant chemotherapy for breast cancer. Int. J. Clin. Oncol. 18, 132–138 (2013).
Nitz, U., Gluz, O., Huober, J., Kreipe, H. H., Kates, R. E., Hartmann, A. et al. Final analysis of the prospective WSG-AGO EC-Doc versus FEC phase III trial in intermediate-risk (pN1) early breast cancer: efficacy and predictive value of Ki67 expression. Ann. Oncol. 25, 1551–1557 (2014).
Ewertz, M., Qvortrup, C. & Eckhoff, L. Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol. 54, 587–591 (2015).
Bandos, H., Melnikow, J., Rivera, D. R., Swain, S. M., Sturtz, K., Fehrenbacher, L. et al. Long-term peripheral neuropathy in breast cancer patients treated with adjuvant chemotherapy: NRG Oncology/NSABP B-30. J. Natl Cancer Inst. 110, https://doi.org/10.1093/jnci/djx162 (2018).
Speck, R. M., Sammel, M. D., Farrar, J. T., Hennessy, S., Mao, J. J., Stineman, M. G. et al. Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J. Oncol. Pract. 9, e234–e240 (2013).
Pachman, D. R., Barton, D. L., Swetz, K. M. & Loprinzi, C. L. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J. Clin. Oncol. 30, 3687–3696 (2012).
Rivera, D. R., Ganz, P. A., Weyrich, M. S., Bandos, H. & Melnikow, J. Chemotherapy-Associated peripheral neuropathy in patients with early-stage breast cancer: a systematic review. J. Natl Cancer Inst. 110, https://doi.org/10.1093/jnci/djx140 (2018).
Seretny, M., Currie, G. L., Sena, E. S., Ramnarine, S., Grant, R., MacLeod, M. R. et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 155, 2461–2470 (2014).
Greenlee, H., Hershman, D. L., Shi, Z., Kwan, M. L., Ergas, I. J., Roh, J. M. et al. BMI, lifestyle factors and taxane-induced neuropathy in breast cancer patients: the Pathways Study. J. Natl Cancer Inst. 109, https://doi.org/10.1093/jnci/djw206 (2017).
Bao, T., Basal, C., Seluzicki, C., Li, S. Q., Seidman, A. D. & Mao, J. J. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res. Treat. 159, 327–333 (2016).
Julian, T., Glascow, N., Syeed, R. & Zis, P. Alcohol-related peripheral neuropathy: a systematic review and meta-analysis. J. Neurol. 266, 2907–2919 (2019).
Hanewinckel, R., van Oijen, M., Ikram, M. A. & van Doorn, P. A. The epidemiology and risk factors of chronic polyneuropathy. Eur. J. Epidemiol. 31, 5–20 (2016).
Rolke, R., Rolke, S., Vogt, T., Birklein, F., Geber, C., Treede, R. D. et al. Hand-arm vibration syndrome: clinical characteristics, conventional electrophysiology and quantitative sensory testing. Clin. Neurophysiol. 124, 1680–1688 (2013).
Heaver, C., Goonetilleke, K. S., Ferguson, H. & Shiralkar, S. Hand-arm vibration syndrome: a common occupational hazard in industrialized countries. J. Hand Surg. Eur. Vol. 36, 354–363 (2011).
Mols, F., van de Poll-Franse, L. V., Vreugdenhil, G., Beijers, A. J., Kieffer, J. M., Aaronson, N. K. et al. Reference data of the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-CIPN20 Questionnaire in the general Dutch population. Eur. J. Cancer 69, 28–38 (2016).
Barlow, L., Westergren, K., Holmberg, L. & Talbäck, M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 48, 27–33 (2009).
Aaronson, N. K., Ahmedzai, S., Bergman, B., Bullinger, M., Cull, A., Duez, N. J. et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J. Natl Cancer Inst. 85, 365–376 (1993).
Zigmond, A. S. & Snaith, R. P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 67, 361–370 (1983).
Postma, T. J., Aaronson, N. K., Heimans, J. J., Muller, M. J., Hildebrand, J. G., Delattre, J. Y. et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur. J. Cancer 41, 1135–1139 (2005).
Kieffer, J. M., Postma, T. J., van de Poll-Franse, L., Mols, F., Heimans, J. J., Cavaletti, G. et al. Evaluation of the psychometric properties of the EORTC chemotherapy-induced peripheral neuropathy questionnaire (QLQ-CIPN20). Qual. Life Res. 26, 2999–3010 (2017).
Cavaletti, G., Cornblath, D. R., Merkies, I. S., Postma, T. J., Rossi, E., Frigeni, B. et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: from consensus to the first validity and reliability findings. Ann. Oncol. 24, 454–462 (2013).
Lavoie Smith, E. M., Barton, D. L., Qin, R., Steen, P. D., Aaronson, N. K. & Loprinzi, C. L. Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual. Life Res. 22, 2787–2799 (2013).
Smith, E. M. L., Banerjee, T., Yang, J. J., Bridges, C. M., Alberti, P., Sloan, J. A. et al. Psychometric Testing of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Chemotherapy-Induced Peripheral Neuropathy 20-Item Scale Using Pooled Chemotherapy-Induced Peripheral Neuropathy Outcome Measures Standardization and Alliance for Clinical Trials in Oncology A151408 Study Data. Cancer Nurs. 42, 179–189 (2019).
Olsson, S. J., Ekblom, Ö., Andersson, E., Börjesson, M. & Kallings, L. V. Categorical answer modes provide superior validity to open answers when asking for level of physical activity: a cross-sectional study. Scand. J. Public Health 44, 70–76 (2016).
Bush, K., Kivlahan, D. R., McDonell, M. B., Fihn, S. D. & Bradley, K. A. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 158, 1789–1795 (1998).
McNutt, L. A., Wu, C., Xue, X. & Hafner, J. P. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am. J. Epidemiol. 157, 940–943 (2003).
Winters-Stone, K. M., Horak, F., Jacobs, P. G., Trubowitz, P., Dieckmann, N. F., Stoyles, S. et al. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J. Clin. Oncol. 35, 2604–2612 (2017).
Mustafa Ali, M., Moeller, M., Rybicki, L. & Moore, H. C. F. Long-term peripheral neuropathy symptoms in breast cancer survivors. Breast Cancer Res. Treat. https://doi.org/10.1007/s10549-017-4437-8 (2017).
Lavoie Smith, E. M., Haupt, R., Kelly, J. P., Lee, D., Kanzawa-Lee, G., Knoerl, R. et al. The content validity of a chemotherapy-induced peripheral neuropathy patient-reported outcome measure. Oncol. Nurs. Forum 44, 580–588 (2017).
Wang, M., Cheng, H. L., Lopez, V., Sundar, R., Yorke, J. & Molassiotis, A. Redefining chemotherapy-induced peripheral neuropathy through symptom cluster analysis and patient-reported outcome data over time. BMC Cancer 19, 1151 (2019).
Molassiotis, A., Cheng, H. L., Lopez, V., Au, J. S. K., Chan, A., Bandla, A. et al. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer 19, 132 (2019).
Frisina, R. D., Wheeler, H. E., Fossa, S. D., Kerns, S. L., Fung, C., Sesso, H. D. et al. Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J. Clin. Oncol. 34, 2712–2720 (2016).
Hanewinckel, R., Drenthen, J., van Oijen, M., Hofman, A., van Doorn, P. A. & Ikram, M. A. Prevalence of polyneuropathy in the general middle-aged and elderly population. Neurology 87, 1892–1898 (2016).
Pérez-Fidalgo, J. A., Roselló, S., García-Garré, E., Jordá, E., Martín-Martorell, P., Bermejo, B. et al. Incidence of chemotherapy-induced amenorrhea in hormone-sensitive breast cancer patients: the impact of addition of taxanes to anthracycline-based regimens. Breast Cancer Res. Treat. 120, 245–251 (2010).
Gonnelli, S. & Petrioli, R. Aromatase inhibitors, efficacy and metabolic risk in the treatment of postmenopausal women with early breast cancer. Clin. Interv. Aging 3, 647–657 (2008).
Neuhouser, M. L., Aragaki, A. K., Prentice, R. L., Manson, J. E., Chlebowski, R., Carty, C. L. et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol. 1, 611–621 (2015).
Hershman, D. L., Till, C., Wright, J. D., Awad, D., Ramsey, S. D., Barlow, W. E. et al. Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in southwest oncology group clinical trials. J. Clin. Oncol. 34, 3014–3022 (2016).
Bhattacharyya, S. & Helfgott, S. M. Neurologic complications of systemic lupus erythematosus, Sjögren syndrome, and rheumatoid arthritis. Semin. Neurol. 34, 425–436 (2014).
Brydøy, M., Oldenburg, J., Klepp, O., Bremnes, R. M., Wist, E. A., Wentzel-Larsen, T. et al. Observational study of prevalence of long-term Raynaud-like phenomena and neurological side effects in testicular cancer survivors. J. Natl Cancer Inst. 101, 1682–1695 (2009).
Jordan, B., Margulies, A., Cardoso, F., Cavaletti, G., Haugnes, H. S., Jahn, P. et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 31, 1306–1319 (2020).
Hershman, D. L., Weimer, L. H., Wang, A., Kranwinkel, G., Brafman, L., Fuentes, D. et al. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res. Treat. 125, 767–774 (2011).
Acknowledgements
We wish to thank all the study participants, Marika Wenemark for assistance in developing the questionnaire and Rasmus Mikiver for cancer register assistance.
Author information
Authors and Affiliations
Contributions
Conceptualisation; data curation; formal analysis; methodology; resources; software; supervision; validation; visualisation; roles/writing—original draft: K.E., M.F., H.G. and E.Å.-L. Funding acquisition; investigation; project administration; writing—review and editing: K.E., H.G. and E.Å.L.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Regional Ethics Committee in Linköping approved the study (Ref. no. 2016/548-31). All women who participated in the study gave informed consent by returning the questionnaire. The study was performed in accordance with the Declaration of Helsinki.
Consent to publish
This study contained no individual person’s data.
Data availability
The dataset analysed during the current study is available from the corresponding author on reasonable request.
Competing interests
The funding sources played no role in the study. The authors declare no competing interests.
Funding information
This work was supported by the Swedish Cancer Society (190224); the Medical Research Council of Southeast Sweden (FORSS-932359); Futurum—The Academy for Health and Care, Jönköping County Council (575361); Forsknings-ALF (LIO-901261).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Engvall, K., Gréen, H., Fredriksson, M. et al. Persistent neuropathy among early-stage breast cancer survivors in a population-based cohort. Br J Cancer 125, 445–457 (2021). https://doi.org/10.1038/s41416-021-01429-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01429-3
This article is cited by
-
Impact of persistent peripheral neuropathy on health-related quality of life among early-stage breast cancer survivors: a population-based cross-sectional study
Breast Cancer Research and Treatment (2022)