Abstract
Background
The effect of Porphyromonas gingivalis (Pg) infection on oesophageal squamous cell carcinoma (ESCC) prognosis, chemotherapeutic efficacy, and oesophageal cancer cell apoptosis resistance and proliferation remain poorly understood.
Methods
Clinicopathological data from 312 ESCC oesophagectomy patients, along with the computed tomography imaging results and longitudinal cancerous tissue samples from a patient subset (n = 85) who received neoadjuvant chemotherapy (NACT), were analysed. Comparison of overall survival and response rate to NACT between Pg-infected and Pg-uninfected patients was made by multivariate Cox analysis and Response Evaluation Criteria in Solid Tumours v.1.1 criteria. The influence of Pg on cell proliferation and drug-induced apoptosis was examined in ESCC patients and validated in vitro and in vivo.
Results
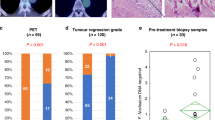
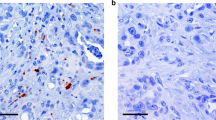
The 5-year overall survival was lower in Pg-positive patients, and infection was associated with multiple clinicopathological factors and pathologic tumour, node, metastasis stage. Of the 85 patients who received NACT, Pg infection was associated with a lower response rate and 5-year overall survival. Infection with Pg resulted in apoptosis resistance in ESCC and promoted ESCC cell viability, which was confirmed in longitudinal cancerous tissue samples. Pg-induced apoptosis resistance was dependent on fimbriae and STAT3.
Conclusions
Pg infection is associated with a worse ESCC prognosis, reduced chemotherapy efficacy, and can potentiate the aggressive behaviour of ESCC cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lichtenstein, P., Holm, N. V., Verkasalo, P. K., Iliadou, A., Kaprio, J., Koskenvuo, M. et al. Environmental and heritable factors in the causation of cancer−analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 343, 78–85 (2000).
Gagnaire, A., Nadel, B., Raoult, D., Neefjes, J. & Gorvel, J. P. Collateral damage: insights into bacterial mechanisms that predispose host cells to cancer. Nat. Rev. Microbiol. 15, 109–128 (2017).
Nagy, R., Sweet, K. & Eng, C. Highly penetrant hereditary cancer syndromes. Oncogene 23, 6445–6470 (2004).
de Martel, C., Ferlay, J., Franceschi, S., Vignat, J., Bray, F., Forman, D. et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 13, 607–615 (2012).
Oh, J. K. & Weiderpass, E. Infection and cancer: global distribution and burden of diseases. Ann. Glob. Health 80, 384–392 (2014).
Gur, C., Ibrahim, Y., Isaacson, B., Yamin, R., Abed, J., Gamliel, M. et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42, 344–355 (2015).
Schiffman, M., Doorbar, J., Wentzensen, N., de Sanjose, S., Fakhry, C., Monk, B. J. et al. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Prim. 2, 16086 (2016).
Whitmore, S. E. & Lamont, R. J. Oral bacteria and cancer. PLoS Pathog. 10, e1003933 (2014).
Yu, T., Guo, F., Yu, Y., Sun, T., Ma, D., Han, J. et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170, 548–563.e516 (2017).
Geller, L. T., Barzily-Rokni, M., Danino, T., Jonas, O. H., Shental, N., Nejman, D. et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017).
Bullman, S., Pedamallu, C. S., Sicinska, E., Clancy, T. E., Zhang, X., Cai, D. et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448 (2017).
Darveau, R. P. Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 8, 481–490 (2010).
Lamont, R. J., Koo, H. & Hajishengallis, G. The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 16, 745–759 (2018).
Hajishengallis, G., Darveau, R. P. & Curtis, M. A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 10, 717–725 (2012).
Hajishengallis, G. & Lamont, R. J. Dancing with the stars: how choreographed bacterial interactions dictate nososymbiocity and give rise to keystone pathogens, accessory pathogens, and pathobionts. Trends Microbiol. 24, 477–489 (2016).
Ohshima, J., Wang, Q., Fitzsimonds, Z. R., Miller, D. P., Sztukowska, M. N., Jung, Y. J. et al. Streptococcus gordonii programs epithelial cells to resist ZEB2 induction by Porphyromonas gingivalis. Proc. Natl Acad. Sci. USA 116, 8544–8553 (2019).
Lamont, R. J. & Hajishengallis, G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol. Med. 21, 172–183 (2015).
Kuboniwa, M., Houser, J. R., Hendrickson, E. L., Wang, Q., Alghamdi, S. A., Sakanaka, A. et al. Metabolic crosstalk regulates Porphyromonas gingivalis colonization and virulence during oral polymicrobial infection. Nat. Microbiol. 2, 1493–1499 (2017).
Blaser, M. J. & Falkow, S. What are the consequences of the disappearing human microbiota? Nat. Rev. Microbiol. 7, 887–894 (2009).
Di Pilato, V., Freschi, G., Ringressi, M. N., Pallecchi, L., Rossolini, G. M. & Bechi, P. The esophageal microbiota in health and disease. Ann. NY Acad. Sci. 1381, 21–33 (2016).
Yang, L., Francois, F. & Pei, Z. Molecular pathways: pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett esophagus. Clin. Cancer Res. 18, 2138–2144 (2012).
Geng, F., Liu, J., Guo, Y., Li, C., Wang, H., Wang, H. et al. Persistente exposure to Porphyromonas gingivalis promotes proliferative and invasion capabilities, and tumorigenic properties of human immortalized oral epithelial cells. Front. Cell Infect. Microbiol. 7, 57 (2017).
Liu, J., Tang, X., Li, C., Pan, C., Li, Q., Geng, F. et al. Porphyromonas gingivalis promotes the cell cycle and inflammatory cytokine production in periodontal ligament fibroblasts. Arch. Oral Biol. 60, 1153–1161 (2015).
Mao, S., Park, Y., Hasegawa, Y., Tribble, G. D., James, C. E., Handfield, M. et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 9, 1997–2007 (2007).
Kuboniwa, M., Hasegawa, Y., Mao, S., Shizukuishi, S., Amano, A., Lamont, R. J. et al. P. gingivalis accelerates gingival epithelial cell progression through the cell cycle. Microbes Infect. 10, 122–128 (2008).
Ahn, J., Segers, S. & Hayes, R. B. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis 33, 1055–1058 (2012).
Ogrendik, M. Periodontal pathogens in the etiology of pancreatic cancer. Gastrointest. Tumors 3, 125–127 (2017).
Zhang, Y. Epidemiology of esophageal cancer. World J. Gastroenterol. 19, 5598–5606 (2013).
Gao, S., Li, S., Ma, Z., Liang, S., Shan, T., Zhang, M. et al. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect. Agents cancer 11, 3 (2016).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Baba, Y., Watanabe, M., Yoshida, N. & Baba, H. Neoadjuvant treatment for esophageal squamous cell carcinoma. World J. Gastrointest. Oncol. 6, 121–128 (2014).
Lv, J., Cao, X. F., Zhu, B., Ji, L., Tao, L. & Wang, D. D. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J. Gastroenterol. 16, 1649–1654 (2010).
Li, S., Liu, H., Diao, C., Wang, X., Gao, M., Li, Z. et al. Prognosis of surgery combined with different adjuvant therapies in esophageal cancer treatment: a network meta-analysis. Oncotarget 8, 36339–36353 (2017).
Bellmunt, J., Pons, F. & Orsola, A. Molecular determinants of response to cisplatin-based neoadjuvant chemotherapy. Curr. Opin. Urol. 23, 466–471 (2013).
Rumiato, E., Boldrin, E., Amadori, A. & Saggioro, D. Predictive role of host constitutive variants in neoadjuvant therapy of esophageal cancer. Pharmacogenomics 17, 805–820 (2016).
Enzinger, P. C. & Mayer, R. J. Esophageal cancer. N. Engl. J. Med. 349, 2241–2252 (2003).
Krajewski, K. M., Nishino, M., Franchetti, Y., Ramaiya, N. H., Van den Abbeele, A. D. & Choueiri, T. K. Intraobserver and interobserver variability in computed tomography size and attenuation measurements in patients with renal cell carcinoma receiving antiangiogenic therapy: implications for alternative response criteria. Cancer 120, 711–721 (2014).
Therasse, P., Arbuck, S. G., Eisenhauer, E. A., Wanders, J., Kaplan, R. S., Rubinstein, L. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 92, 205–216 (2000).
Yilmaz, O., Watanabe, K. & Lamont, R. J. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell. Microbiol. 4, 305–314 (2002).
Salic, A. & Mitchison, T. J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl Acad. Sci. USA 105, 2415–2420 (2008).
Gao, S., Li, S., Duan, X., Gu, Z., Ma, Z., Yuan, X. et al. Inhibition of glycogen synthase kinase 3 beta (GSK3beta) suppresses the progression of esophageal squamous cell carcinoma by modifying STAT3 activity. Mol. Carcinog. https://doi.org/10.1002/mc.22685 (2017).
Lu, L., Yakoumatos, L., Ren, J., Duan, X., Zhou, H., Gu, Z. et al. JAK3 restrains inflammatory responses and protects against periodontal disease through Wnt3a signaling. FASEB J. 34, 9120–9140 (2020).
Qi, Y. J., Jiao, Y. L., Chen, P., Kong, J. Y., Gu, B. L., Liu, K. et al. Porphyromonas gingivalis promotes progression of esophageal squamous cell cancer via TGFbeta-dependent Smad/YAP/TAZ signaling. PLoS Biol. 18, e3000825 (2020).
Austin, P. C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 46, 399–424 (2011).
Austin, P. C. & Stuart, E. A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 34, 3661–3679 (2015).
Weinberg, A., Belton, C. M., Park, Y. & Lamont, R. J. Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect. Immun. 65, 313–316 (1997).
Zhang, W., Ju, J., Rigney, T. & Tribble, G. D. Fimbriae of Porphyromonas gingivalis are important for initial invasion of osteoblasts, but not for inhibition of their differentiation and mineralization. J. Periodontol. 82, 909–916 (2011).
Yao, L., Jermanus, C., Barbetta, B., Choi, C., Verbeke, P., Ojcius, D. M. et al. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol. Oral Microbiol. 25, 89–101 (2010).
Boisvert, H. & Duncan, M. J. Translocation of Porphyromonas gingivalis gingipain adhesin peptide A44 to host mitochondria prevents apoptosis. Infect. Immun. 78, 3616–3624 (2010).
Pei, Z., Bini, E. J., Yang, L., Zhou, M., Francois, F. & Blaser, M. J. Bacterial biota in the human distal esophagus. Proc. Natl Acad. Sci. USA 101, 4250–4255 (2004).
Yang, L., Lu, X., Nossa, C. W., Francois, F., Peek, R. M. & Pei, Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 137, 588–597 (2009).
Pei, Z., Yang, L., Peek, R. M. Jr, Levine, S. M., Pride, D. T. & Blaser, M. J. Bacterial biota in reflux esophagitis and Barrett’s esophagus. World J. Gastroenterol. 11, 7277–7283 (2005).
Acknowledgements
We acknowledge Drs. Mi and Zhang for assisting in the pathological evaluation and for other technical advice.
Author information
Authors and Affiliations
Contributions
H.W., S.G., and F.Z conceived the study. Y.L., K.L., X.D., M.M., Z.G., L.Y., and J.R. performed most of the experiments and interpreted the data. X.Y., S.L., D.A.S., R.J.L., and H.W. directed the study and supervised the research. Y.L. and K.L. collected tumour specimens and analysed the clinical data of patients. X.D., J.R., and Z.G. performed all immunofluorescence staining. Y.L. performed animal experiments. S.G., F.Z., and H.W. confirmed the histopathological findings and interpreted the clinical data. H.W. prepared the manuscript. D.A.S. and R.J.L. reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Tissue samples and clinicopathological data were obtained from The First Affiliated Hospital of Henan University of Science and Technology and Anyang People’s Hospital. The research was approved by the Research Ethics Committee of Henan University of Science and Technology and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent from each patient was achieved. All animal experiments were authorised through the Animal Care and Use Committee of Henan University of Science and Technology (HAUST). All animal experiments were conducted in accordance with the Guidelines for Animal Health and Use of Henan University of Science and Technology (HAUST).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Funding information
This research was supported by grants DE026727 (H.W.), DE017921, DE011111 (R.J.L.), and DE017680 (D.A.S.) from National Institute of Dental and Craniofacial Research, NIH, USA, and by the Natural Science Foundation of China (NSFC, GS 81472234), and Key Programs of Science and Technology of Henan Province (KPST-HN, GS 161100311200).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gao, S., Liu, Y., Duan, X. et al. Porphyromonas gingivalis infection exacerbates oesophageal cancer and promotes resistance to neoadjuvant chemotherapy. Br J Cancer 125, 433–444 (2021). https://doi.org/10.1038/s41416-021-01419-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01419-5