Abstract
Background
Microwave ablation (MWA) is an effective minimally invasive technique for lung tumours. We aim to evaluate its role for pulmonary oligorecurrence after radical surgery of non-small-cell lung cancer (NSCLC).
Methods
From June 2012 to Jan 2020, a total of 103 patients with pulmonary oligorecurrence after previous radical surgical resection of NSCLC were retrospectively analysed. The primary endpoint was postoperative progression-free survival (PFS). Secondary endpoints were postoperative overall survival (OS), patterns of failure, complications and predictive factors associated with prognosis.
Results
Of the 103 patients identified, 135 pulmonary oligorecurrences developed at a median interval of 34.8 months. In total, 143 sessions of MWA were performed to ablate all the nodules. The median PFS and OS were 15.1 months and 40.6 months, respectively. After MWA, 15 (14.6%) patients had local recurrence as the first event, while intrathoracic oligorecurrence and distant metastases were observed in 45 (43.7%) and 20 (19.4%) patients, respectively. In the multivariate analysis, local recurrence and intrathoracic oligorecurrence were not significant predictors for OS (P = 0.23 and 0.26, respectively). However, distant metastasis was predictive of OS (HR = 5.37, 95% CI, 1.04–27.84, P = 0.04).
Conclusion
MWA should be considered to be an effective and safe treatment option for selected patients with pulmonary oligorecurrence after NSCLC radical surgical resection.
Similar content being viewed by others
Background
Radical surgical resection remains as the standard treatment for early-stage non-small-cell lung cancer (NSCLC). However, postoperative recurrence occurred in 30–75% of these patients, making it the main obstacle for long-term survival after radical surgical resection.1,2 Post-recurrence survival time was reported to be poor, with a range of 8–14 months,3,4 and the 5-year post-recurrence survival rate was only 13%.5 Standard treatment for postoperative recurrence of NSCLC remains controversial. Systemic chemotherapy and/or molecular targeted therapy is commonly applied for postoperative recurrent NSCLCs; however, recent studies indicated that effective and aggressive local treatment may benefit for a substantial proportion of patients that experience postoperative oligorecurrence. Oligorecurrence is an intermediate state between locally confined recurrence and widespread distant metastasis, in which recurrent lesions are limited in number and location (usually ≤ 5).6,7 For selected NSCLC patients with postoperative oligorecurrence, effective local therapy, including secondary surgical resection or radiotherapy, has been shown to achieve long-term survival or even cure.8,9,10,11
Image-guided percutaneous microwave ablation (MWA) as a newly developed minimally invasive technique in the past decades can result in good local control, safety and survival for primary and metastatic lung tumours.12,13,14 Furthermore, our recently published results indicated that MWA may serve as an effective treatment option for stage IV NSCLCs with synchronous extracranial oligometastasis.15 We hypothesise that MWA may also lead to good efficacy in NSCLC patients with postoperative pulmonary oligorecurrence. The present multicentre study was initiated to summarise our experience of MWA for postoperative pulmonary oligorecurrent lesions after complete resection of NSCLC.
Methods
Patient selection
From June 2012 to Jan 2020, 103 patients with pulmonary oligorecurrence after previous radical R0 surgical resection of histologically proven NSCLC that met all inclusion criteria were eligible to enrol in this retrospective study (patients’ selection steps shown in Fig. 1). The ethics committee of each institute approved this study. In this study, pulmonary oligorecurrence was defined as five or fewer local or metastatic nodules after surgical resection confined only to the lung (both ipsilaterally and contralaterally). The inclusion criteria for the study were as follows: (1) initial radical (R0) resection of histologically proven NSCLC, (2) patients with pulmonary oligorecurrences, (3) all recurrent lesions underwent completely microwave ablation, (4) ≥18 years old, Eastern Cooperative Oncology Group (ECOG) performance status (PS) score ≤2 (5) and <10 years between initial surgery and MWA.
The exclusion criteria included (1) patients without a systemic examination prior to enrolling in this study, (2) <6 months of follow-up after MWA intervention (excluding death cases), (3) presence of concurrent mediastinal and hilar lymph node recurrence, (4) presence of metastases to organs excluding lung or pleural seeding, (5) presence of second primary lung cancers6 and metachronous intrapulmonary nodules with ground-glass attenuation on chest CT suspected as second primary lung cancers but histologically unproved.
Histological or cytological confirmation of the diagnosis was made when clinically feasible. Radiological evidence of recurrent lesion was accepted if the biopsy was not feasible. The criteria by Martini and Melamad16 were utilised for the differential diagnosis between second primary lung cancers and intrapulmonary recurrences. Clinical data collected from each patient included age, gender, the interval between surgery and recurrence (disease-free survival (DFS)), recurrence sites and numbers.
Treatment
The decision on diagnosis and MWA treatment plan of postoperative pulmonary oligorecurrences, was made by the institutional multidisciplinary board of lung cancer based on patients’ general conditions, cardiopulmonary functions and distributions of metastasis. Routine laboratory tests, lung function tests and radiological examinations, including chest, abdomen and pelvic-enhanced CT, and brain magnetic resonance imaging (MRI), were performed before lung MWA ablation in all cases. Detailed ablation procedures were as we previously described.17 For tumours located under the pleura or near the chest wall, subpleural anaesthesia and/or artificial pneumothorax was applied. Furthermore, simultaneous multi-antenna MWA was performed for tumours that were over 3 cm to increase the ablation zone. Lesions in both lungs were ablated separately within one month. The administration of adjuvant chemotherapy, TKIs or immunotherapy, was allowed after MWA intervention if necessary. Patients received contrast-enhanced computed tomography (CT) to determine the local response (complete or incomplete ablation) one month after ablation.
Follow-up evaluations
Patients’ follow-up consisted of contrast-enhanced CT one month after MWA, then every 3 months for the first year, then every 6 months from the 2nd to the 5th year and annually thereafter. Local recurrence was defined as a progressive enlarged enhanced nodule in the margin of ablation field or new lesions that occurred in the same lobe.18 PET-CT was also utilised for undefined nodules when necessary. Intrathoracic oligorecurrence and distant metastases were also documented during follow-up. Intrathoracic oligorecurrence was defined as less than five recurrences limited in thoracic cavity excluding the previously ablated pulmonary lobes. Over five intrathoracic recurrent lesions or extrathoracic metastases were classified as distant metastases.
Statistical analysis
The primary endpoint was progression-free survival (PFS), which was calculated from the beginning date of pulmonary MWA to the date of any new recurrence. Secondary endpoints were overall survival (OS), patterns of failure, toxicity and predictive factors associated with prognosis. OS was calculated from the beginning date of pulmonary MWA to the date of death or the last follow-up. Patients known to be alive at last contact were censored. Categorical data were compared with χ2 test, while continuous data were compared with t tests. The Kaplan–Meier log-rank test was used for survival curve comparisons. Univariate and multivariate Cox proportional hazard models were performed to screen prognostic factors and the corresponding hazard ratios (HR) for different factors with 95% confidence intervals (CI). P values were two-sided and considered significant if <0.05. Statistical analysis was performed using SPSS for Windows Version 17.0 (IBM, Chicago, IL).
Results
Patient characteristics
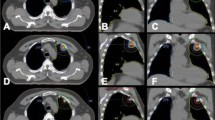
Based on our inclusion and exclusion criteria, 103 patients with 135 post-surgery pulmonary oligorecurrent nodules were enrolled in the present study (patients’ characteristics summarised in Table 1). Of these, solitary pulmonary recurrence was most common (71/103, 68.9%), followed by two (21/103, 20.4%), three (10/103, 9.7%) and four (1/103, 1.0%) recurrent lesions. In total, 143 sessions of ablation were performed (an example case shown in Fig. 2). The initial pulmonary resection was standard surgery (lobectomy/pneumonectomy with mediastinal lymph node dissection) in 97/103 patients (94.2%), while wedge resection was performed in 6 patients. All patients received enhanced CT staging, and biopsies of 96 recurrent tumours (71.1%) were performed to confirm the same histologic type as prior lung cancers. Pathological diagnosis at primary surgery was adenocarcinoma in 69 patients (67.0%), squamous carcinoma in 26 (25.2%) and other pathological types in 8 patients (7.8%). The median interval from initial surgery to recurrence (DFS) was 34.8 months (ranging from 3.0 to 108.6 months). The mean diameter of all metastatic lesions was 1.77 cm (range from 0.6 to 3.9 cm), and mean distance to the pleural surface was 2.14 cm (range from 0 to 5.8 cm). In all cases, 61 (59.2%) of them received adjuvant therapy after MWA.
Chest CT scans showing postoperative oligorecurrences (arrows) in the left (a) and right (b) lung. c CT scan during the MWA of the left pulmonary metastasis. d The patient received right pulmonary metastasis MWA at 1-week interval. e–h Follow-up enhanced CT obtained one month after MWA showing the ablation zones. i–l Follow-up enhanced CT obtained 18 months after MWA showing gradual constriction of the ablation zone and no local recurrence.
Patterns of failure and outcomes after pulmonary MWA
The median follow-up period after MWA was 31.8 months (range, 3.6–76.6 months). During follow-up, 80 (77.6%) patients experienced disease progression after pulmonary MWA and the median PFS was 15.1 months (range, 1.2–70.6 months). Fifteen (14.6%) patients had local recurrence as the first event, while intrathoracic oligorecurrence and distant metastases were observed in 45 (43.7%) and 20 (19.4%), respectively. All the local recurrent lesions were controlled by repeated pulmonary MWA (an example case shown in Fig. 3), and most (42, 93%) patients with intrathoracic oligorecurrence also received secondary ablation. However, MWA was not applied in patients who developed distant metastases. For all cases, the estimated PFS rate was 64.1% at 1 year, 24.4% at 3 years and 9.2% at 5 years (Fig. 4a). The median OS was 40.6 months and the estimated OS rate was 97.1% at 1 year, 58.7% at 3 years and 34.3% at 5 years (Fig. 4b). If calculating from the date of primary tumour resection, the median OS reached 93.7 months and the estimated survival rate was 88.2% at 3 years, 74.5% at 5 years and 27.5% at 10 years, respectively.
a, b Enhanced chest CT scan before MWA showing a solitary metastatic nodule in the left lower lobe. c CT scan obtained during MWA. d, e Follow-up CT scans obtained 3 months after the MWA. f, g The ablation zone gradually enlarged 7 months after the MWA and was considered as local recurrence. h, i A secondary MWA was performed to ablate the local recurrence. Double antennas were simultaneously inserted to cover the lesion. j–l Follow-up CT scans obtained 1 month (j), 3 months (k) and 10 months (l) after MWA showing gradual constriction of the ablation zone and no evidence of local recurrence.
Prognostic factors for survival
We next examine the potential prognostic factors for PFS and OS by Cox regression analysis (Table 2). Age, gender, DFS or adjuvant treatment was not identified as significant prognostic factors. In both univariate and multivariate analysis, adenocarcinoma and lower primary tumour staging was significantly associated with better PFS after MWA intervention. Patients with adenocarcinoma had longer PFS (median: 24.9 vs. 8.0 months, HR = 0.29, 95% CI 0.16–0.51) than those with non-adenocarcinoma pathological diagnosis (Fig. 5a). Stage I disease had significantly better PFS than stage II–III disease (median: 15.0 vs. 15.1 months, HR = 0.49, 95% CI 0.31–0.77) (Fig. 5b). The impact of adenocarcinoma on OS was not obvious in multivariate analysis. Notably, staging was a prognostic factor correlated with not only PFS but also OS. The OS of patients with stage I disease was significantly longer than those with stage II–III disease (median: 61.6 vs. 36.8 months, HR = 0.46, 95% CI 0.26–0.83) (Fig. 5c). Moreover, patterns of failure were also associated with OS. In comparison with patients without recurrence or metastasis, OS was remarkably worse in patients that developed secondary progression (median: 31.2 vs. undefined, HR = 3.68, 95% CI 2.10–6.46) (Fig. 5d). In the multivariate analysis, local recurrence and intrathoracic oligorecurrence were not significant predictors for OS (P = 0.23 and 0.26, respectively). However, distant metastasis was predictive of OS (HR = 5.37, 95% CI, 1.04–27.84, P = 0.04).
a PFS curves by pathology: adenocarcinoma had longer PFS (median: 24.9 vs. 8.0 months, HR = 0.29, 95% CI 0.16–0.51) than non-adenocarcinoma. b PFS curves by stage: The PFS of Stage I was significantly better than stage II–III (median: 15.0 vs. 15.1 months, HR = 0.49, 95% CI 0.31–0.77). c OS curves by stage: The OS of Stage I was significantly better than stage II–III (median: 61.6 vs. 36.8 months, HR = 0.46, 95% CI 0.26–0.83). d OS by patterns of failure: OS was remarkably worse in patients that developed secondary progression (local recurrence + intrathoracic oligorecurrence + distant metastases) (median: 31.2 vs. undefined, HR = 3.68, 95% CI 2.10–6.46). Distant metastasis was predictive of OS (HR = 5.37, 95% CI, 1.04–27.84, P = 0.04).
Complications
No death occurred during ablation procedures or within 30 days after MWA. Pneumothorax developed in 43 of 143 MWA sessions (30.1%), while 9 of them required chest tube drainage. In total, 62 (60.2%) patients suffered mild or moderate pain after ablation and can be managed by non-opioid analgesics. Self-limiting pulmonary haemorrhage/haemoptysis and pleural effusion occurred in 23 (16.1%) and 9 (6.3%) sessions, respectively. Post-ablation invasive pulmonary aspergillosis was observed in two (1.4%) sessions and successfully controlled by voriconazole. Bronchopleural fistula occurred in one (0.7%) patient and thoracic tube drainage was required. Self-limited post-ablation syndrome, including fever (below 38.5 °C), fatigue, general malaise, nausea and vomiting, occurred in 22 (15.4%) of ablation sessions.
Discussion
This clinical study identified that MWA can lead to excellent local control and long-term survival in NSCLC patients with postoperative pulmonary oligorecurrences. Currently, standard treatment for NSCLCs with post-operation pulmonary recurrence is still controversial. Accumulating evidence showed that aggressive local therapy, including secondary surgery and radiotherapy for solitary or a limited number of metastatic lesions (termed oligorecurrence), may predict a favourable post-recurrence survival or even cure. In a small-sample study with 16 patients that developed a solitary postoperative pulmonary recurrence, second surgical resection was associated with long-term survival.19 More recently, Han et al. reported that the post-recurrence survival was significantly longer in patients with pulmonary oligorecurrence who received operative treatment than those with non-operative therapy.9 In addition, postoperative salvage stereotactic body radiotherapy (SBRT) showed good efficacy and tolerance in NSCLC patients with intrathoracic oligorecurrence.20,21 However, the result of thermal ablation for the treatment of oligorecurrent NSCLC after surgical resection is still limited. Kodama et al. reported a 1-, 3- and 5-year OS rate of 97.7, 72.9 and 55.7% in a series with 51 patients who received lung radiofrequency ablation (RFA) for unresectable recurrences after surgical intervention.22 In the present study, we identified that MWA treatment for the pulmonary oligorecurrences was associated with a median OS of 40.6 months, and the 1-, 3- and 5-year survival rate was 97.1%, 58.7% and 34.3%, respectively. These results are comparable with previous reports of RFA,22 or second resection.9 Considering that most of these patients were medically inoperable, the outcomes of MWA for the management of post-operation pulmonary oligorecurrence are promising.
The most common treatment failure after pulmonary MWA we observed was intrathoracic oligorecurrence (45/103, 43.7%), followed by distant metastases (20/103, 19.4%) and local recurrence (15/103, 14.6%). Although it is difficult to determine whether metastasis events were due to pulmonary MWA treatment failure or pre-existing distant seeding from the prior tumour, patients with local or distant failure had significantly worse OS than patients that did not develop recurrence. In addition, patients that developed local recurrence or intrathoracic oligorecurrence had better survival than those with distant metastases, while the difference of survival was not significant if recurrence was limited in the lung. Noteworthy, most of these patients received secondary ablation for their recurrent lesions, indicating the efficacy and safety of repeated MWA in this instance. We subsequently investigated other prognostic factors associated with PFS and OS to understand which patients will fare better with pulmonary oligorecurrence MWA. In multivariate analysis, adenocarcinoma was associated with better PFS, while the lower pathological stage was correlated with both favourable PFS and OS. These results indicated that initial tumour burden was still an important marker of poor response and long-term survival for NSCLCs that developed pulmonary oligorecurrence after radical surgery intervention.
We found that the majority of complications by MWA were mild and well-tolerated. Pneumothorax was one of the most common major complications. It was reported that previous pulmonary surgery may be a risk factor for pneumothorax.23 The incidence of pneumothorax in this study was 30.1%, and 20.9% of them required chest tube drainage, similar to that we previously reported in primary lung cancer ablation.24,25 As a relatively rare complication, invasive pulmonary aspergillosis was observed in 1.4% sessions, and all cases were cured by administration of voriconazole. The aetiology and high-risk factors of invasive pulmonary aspergillosis for lung MWA are still not clear;26 hence early diagnosis after MWA is critical for patient prognosis.
This study still has several limitations. The first is its retrospective nature. The second is that we did not perform biopsies for all lung nodules to strictly distinguish between the NSCLC pulmonary recurrences and the development of second primary lung cancers. Third, the patient enrolled in the present study is a highly selected population, with limited peripheral lung lesions that are suitable for MWA. Lesions adjacent to big vessels or central lesions are excluded owing to the high risk of pulmonary puncture and post-ablation complications. Moreover, without a control group that included patients who received other local ablative therapies such as surgery, SBRT or RFA, it is difficult to ascertain whether MWA actually improves survival.
In conclusion, we present one of the largest studies of MWA for the treatment of pulmonary oligorecurrences after NSCLC radical surgery intervention. Lung MWA is safe and provides acceptable disease control and survival for patients with either NSCLC oligorecurrences or possibly second primary NSCLCs. Therefore, MWA should be considered as an alternative therapy strategy for postoperative pulmonary oligorecurrent lesions after complete resection of NSCLC. Further large-scale, multicentre, prospective clinical trials are needed to evaluate the role of MWA in postoperative pulmonary oligorecurrent NSCLC.
References
Martin, J., Ginsberg, R. J., Venkatraman, E. S., Bains, M. S., Downey, R. J., Korst, R. J. et al. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J. Clin. Oncol. 20, 1989–1995 (2002).
Takahashi, Y., Horio, H., Hato, T., Harada, M., Matsutani, N. & Kawamura, M. Predictors of post-recurrence survival in patients with non-small-cell lung cancer initially completely resected. Interact. Cardiovasc. Thorac. Surg. 21, 14–20 (2015).
Yoshino, I., Yohena, T., Kitajima, M., Ushijima, C., Nishioka, K., Ichinose, Y. et al. Survival of non-small cell lung cancer patients with postoperative recurrence at distant organs. Ann. Thorac. Cardiovasc. Surg. 7, 204–209 (2001).
Williams, B. A., Sugimura, H., Endo, C., Nichols, F. C., Cassivi, S. D., Allen, M. S. et al. Predicting postrecurrence survival among completely resected nonsmall-cell lung cancer patients. Ann. Thorac. Surg. 81, 1021–1027 (2006).
Sekihara, K., Hishida, T., Yoshida, J., Oki, T., Omori, T., Katsumata, S. et al. Long-term survival outcome after postoperative recurrence of non-small-cell lung cancer: who is ‘cured’ from postoperative recurrence? Eur. J. Cardiothorac. Surg. 52, 522–528 (2017).
Patel, P. R., Yoo, D. S., Niibe, Y., Urbanic, J. J. & Salama, J. K. A call for the aggressive treatment of oligometastatic and oligo-recurrent non-small cell lung cancer. Pulm. Med. 2012, 480961 (2012).
Huang, F., Wu, G. & Yang, K. Oligometastasis and oligo-recurrence: more than a mirage. Radiat. Oncol. 9, 230 (2014).
Hishida, T., Yoshida, J., Aokage, K., Nagai, K. & Tsuboi, M. Postoperative oligo-recurrence of non-small-cell lung cancer: clinical features and survival†. Eur. J. Cardiothorac. Surg. 49, 847–853 (2016).
Han, S. J., Cho, S., Yum, S., Kim, K. & Jheon, S. Surgical treatment of pulmonary oligorecurrence after curative resection for non-small-cell lung cancer. Interact. Cardiovasc Thorac. Surg. 30, 18–23 (2020).
Yuan, Q., Wang, W., Zhang, Q., Wang, Y., Chi, C. & Xu, C. Clinical features and prognostic factor of thoracic postoperative oligo-recurrence of non-small-cell lung cancer. Cancer Manag Res. 12, 1397–403. (2020).
Sun, B., Brooks, E. D., Komaki, R., Liao, Z., Jeter, M., McAleer, M. et al. Long-term outcomes of salvage stereotactic ablative radiotherapy for isolated lung recurrence of non-small cell lung cancer: a phase II clinical trial. J. Thorac. Oncol. 12, 983–92. (2017).
Yang, X., Ye, X., Zheng, A., Huang, G., Ni, X., Wang, J. et al. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J. Surg. Oncol. 110, 758–763 (2014).
Yang, X., Ye, X., Huang, G., Han, X., Wang, J., Li, W. et al. Repeated percutaneous microwave ablation for local recurrence of inoperable Stage I nonsmall cell lung cancer. J. Cancer Res. Ther. 13, 683–688 (2017).
Vogl, T. J., Nour-Eldin, N. A., Albrecht, M. H., Kaltenbach, B., Hohenforst-Schmidt, W., Lin, H. et al. Thermal ablation of lung tumors: focus on microwave ablation. Rofo 189, 828–843 (2017).
Ni, Y., Ye, X., Yang, X., Huang, G., Li, W., Wang, J. et al. Microwave ablation for non-small cell lung cancer with synchronous solitary extracranial metastasis. J. Cancer Res. Clin. Oncol. 146, 1361–1367 (2020).
Martini, N. & Melamed, M. R. Multiple primary lung cancers. J. Thorac. Cardiovasc. Surg. 70, 606–612 (1975).
Ni, Y., Ye, X., Yang, X., Huang, G., Li, W., Wang, J. et al. Microwave ablation as local consolidative therapy for patients with extracranial oligometastatic EGFR-mutant non-small cell lung cancer without progression after first-line EGFR-TKIs treatment. J. Cancer Res. Clin. Oncol. 146, 197–203 (2020).
Ye, X., Fan, W., Wang, H., Wang, J., Wang, Z., Gu, S. et al. Expert consensus workshop report: guidelines for thermal ablation of primary and metastatic lung tumors (2018 edition). J. Cancer Res. Ther. 14, 730–744 (2018).
Hishida, T., Nagai, K., Yoshida, J., Nishimura, M., Ishii, G., Iwasaki, M. et al. Is surgical resection indicated for a solitary non-small cell lung cancer recurrence? J. Thorac. Cardiovasc. Surg. 131, 838–842 (2006).
Aoki, S., Yamashita, H., Takahashi, W., Nawa, K., Ota, T., Imae, T. et al. Salvage stereotactic body radiotherapy for post-operative oligo-recurrence of non-small cell lung cancer: a single-institution analysis of 59 patients. Oncol. Lett. 19, 2695–704. (2020).
Nishiyama, K., Kodama, K., Teshima, T. & Tada, H. Stereotactic body radiotherapy for second pulmonary nodules after operation for an initial lung cancer. Jpn. J. Clin. Oncol. 45, 947–952 (2015).
Kodama, H., Yamakado, K., Takaki, H., Kashima, M., Uraki, J., Nakatsuka, A. et al. Lung radiofrequency ablation for the treatment of unresectable recurrent non-small-cell lung cancer after surgical intervention. Cardiovasc. Interv. Radiol. 35, 563–569 (2012).
Okuma, T., Matsuoka, T., Yamamoto, A., Oyama, Y., Toyoshima, M., Nakamura, K. et al. Frequency and risk factors of various complications after computed tomography-guided radiofrequency ablation of lung tumors. Cardiovasc. Interv. Radiol. 31, 122–130 (2008).
Wang, J., Ni, Y., Yang, X., Huang, G., Wei, Z., Li, W. et al. Diagnostic ability of percutaneous core biopsy immediately after microwave ablation for lung ground-glass opacity. J. Cancer Res. Ther. 15, 755–759 (2019).
Wei, Z., Yang, X., Ye, X., Huang, G., Li, W., Han, X. et al. Camrelizumab combined with microwave ablation improves the objective response rate in advanced non-small cell lung cancer. J. Cancer Res. Ther. 15, 1629–1634 (2019).
Huang, G., Ye, X., Yang, X., Wang, C., Zhang, L., Ji, G. et al. Invasive pulmonary aspergillosis secondary to microwave ablation: a multicenter retrospective study. Int. J. Hyperth. 35, 71–78 (2018).
Author information
Authors and Affiliations
Contributions
Y.N. and X.Ye designed the study, analysed the data and wrote the paper. J.C. and X.L. analysed the data and wrote the paper. J.P., B.Z., X.Yang and Z.W. collected the data.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The ethics committee of Shandong Provincial Hospital Affiliated to Shandong First Medical University, Renji Hospital of Jiaotong University, The First Affiliated Hospital of Shandong First Medical University, Beijing Hospital and National Geriatric Medical Center approved the study. The study was performed in accordance with the Declaration of Helsinki. Informed consent was obtained from the subjects prior to participating in the study.
Consent to publish
Not applicable.
Data availability
All the data generated in this study are included in this paper. The data presented in this paper will be available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Funding information
This study has received funding from National Natural Science Foundation of China (81502610 and 82072028) and Shandong Provincial Natural Science Foundation, China (ZR201911040313).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ni, Y., Peng, J., Yang, X. et al. Multicentre study of microwave ablation for pulmonary oligorecurrence after radical resection of non-small-cell lung cancer. Br J Cancer 125, 672–678 (2021). https://doi.org/10.1038/s41416-021-01404-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01404-y
This article is cited by
-
Clinical outcomes of percutaneous microwave ablation for pulmonary oligometastases from hepatocellular carcinoma: a retrospective, multicenter study
Cancer Imaging (2024)
-
Cone-beam computed tomography image-guided percutaneous microwave ablation for lung nodules in a hybrid operating room: an initial experience
European Radiology (2023)