Abstract
Background
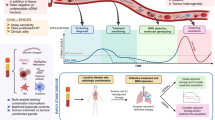
Within the OMITERC prospective study (OMIcs application from solid to liquid biopsy for a personalised ThERapy of Cancer), we explored the prognostic role of liquid biopsy encompassing cell-free DNA (cfDNA) and circulating tumour cells (CTCs) in KRAS mutated metastatic colorectal cancer (mCRC).
Methods
We defined a workflow including pre-analytical and analytical procedures collecting blood before therapy and every 3 months until disease progression (PD). CTCs were counted by CellSearch® and isolated by DEPArray™. NGS sequencing of CTCs and cfDNA was performed using a panel of cancer/CRC related genes respectively.
Results
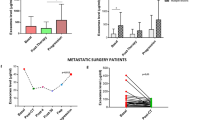
KRAS mutational status was mostly concordant between tumour tissues and liquid biopsy. The percentage of cfDNA samples with mutations in CRC driver genes was in line with literature. In longitudinal monitoring circulating biomarkers anticipated or overlapped conventional diagnostic tools in predicting PD. The presence of CTCs at baseline was confirmed a negative prognostic marker.
Conclusions
Cell-free DNA and CTCs are readily available candidates for clinical application in mCRC. While CTCs demonstrated a prognostic significance at baseline, cfDNA was confirmed an easily accessible material for monitoring the mutational status of the tumour over time. Moreover, in the longitudinal study, the two markers emerged as complementary in assessing disease progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zavridou, M., Mastoraki, S., Strati, A., Tzanikou, E., Chimonidou, M. & Lianidou, E. Evaluation of preanalytical conditions and implementation of quality control steps for reliable gene expression and DNA methylation analyses in liquid biopsies. Clin. Chem. 64, 1522–1533 (2018).
Alix-Panabières, C. & Pantel, K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 6, 479–491 (2016).
Neumann, M. H. D., Bender, S., Krahn, T. & Schlange, T. ctDNA and CTCs in liquid biopsy—current status and where we need to progress. Comput. Struct. Biotechnol. J. 16, 190–195 (2018).
Salvianti, F., Rotunno, G., Galardi, F., De Luca, F., Pestrin, M., Vannucchi, A. M. et al. Feasibility of a workflow for the molecular characterization of single cells by next generation sequencing. Biomol. Detect. Quantif. 5, 23–29 (2015).
De Luca, F., Rotunno, G., Salvianti, F., Galardi, F., Pestrin, M., Gabellini, S. et al. Mutational analysis of single circulating tumor cells by next generation sequencing in metastatic breast cancer. Oncotarget 7, 26107–26109 (2016).
Molnár, B., Galamb, O., Kalmár, A., Barták, B. K., Nagy, Z. B., Tóth, K. et al. Circulating cell-free nucleic acids as biomarkers in colorectal cancer screening and diagnosis—an update. Expert. Rev. Mol. Diagn. 19, 477–498 (2019).
Cohen, S. J., Punt, C. J., Iannotti, N., Saidman, B. H., Sabbath, K. D., Gabrail, N. Y. et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 3213–3221 (2008).
Tol, J., Koopman, M., Miller, M. C., Tibbe, A., Cats, A., Creemers, G. J. et al. Circulating tumour cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann. Oncol. 21, 1006–1012 (2010).
Bidard, F. C., Ferrand, F. R., Huguet, F., Hammel, P., Louvet, C., Malka, D. et al. Disseminated and circulating tumor cells in gastrointestinal oncology. Crit. Rev. Oncol. Hematol. 82, 103–115 (2012).
Raimondi, C., Nicolazzo, C., Gradilone, A., Giannini, G., De Falco, E., Chimenti, I. et al. Circulating tumor cells: exploring intratumor heterogeneity of colorectal cancer. Cancer Biol. Ther. 15, 496–503 (2014).
Salvianti, F., Pazzagli, M. & Pinzani, P. Single circulating tumor cell sequencing as an advanced tool in cancer management. Expert. Rev. Mol. Diagn. 16, 51–63 (2016).
Salvianti, F. & Pinzani, P. The diagnostic potential of mutation detection from single circulating tumor cells in cancer patients. Expert. Rev. Mol. Diagn. 17, 975–981 (2017).
Fehm, T. N., Meier-Stiegen, F., Driemel, C., Jäger, B., Reinhardt, F., Naskou, J. et al. Diagnostic leukapheresis for CTC analysis in breast cancer patients: CTC frequency, clinical experiences and recommendations for standardized reporting. Cytom. A. 93, 1213–1219 (2018).
Kin, C., Kidess, E., Poultsides, G. A., Visser, B. C. & Jeffrey, S. S. Colorectal cancer diagnostics: biomarkers, cell-free DNA, circulating tumor cells and defining heterogeneous populations by single-cell analysis. Expert. Rev. Mol. Diagn. 13, 581–599 (2013).
Huang, D., Sun, W., Zhou, Y., Li, P., Chen, F., Chen, H. et al. Mutations of key driver genes in colorectal cancer progression and metastasis. Cancer Metastasis Rev. 37, 173–187 (2018).
Boussios, S., Ozturk, M. A., Moschetta, M., Karathanasi, A., Zakynthinakis-Kyriakou, N., Katsanos, K. H. et al. The developing story of predictive biomarkers in colorectal cancer. J. Pers. Med. 9, E12 (2019).
Rosty, C., Young, J. P., Walsh, M. D., Clendenning, M., Sanderson, K., Walters, R. J. et al. PIK3CA activating mutation in colorectal carcinoma: associations with molecular features and survival. PLoS ONE 8, e65479 (2013).
McCarthy, A. J. & Chetty, R. Smad4/DPC4. J. Clin. Pathol. 71, 661–664 (2018).
Korphaisarn, K., Morris, V. K., Overman, M. J., Fogelman, D. R., Kee, B. K., Raghav, K. P. S. et al. FBXW7 missense mutation: a novel negative prognostic factor in metastatic colorectal adenocarcinoma. Oncotarget 8, 39268–39279 (2017).
Chang, C. C., Lin, H. H., Lin, J. K., Lin, C. C., Lan, Y. T., Wang, H. S. et al. FBXW7 mutation analysis and its correlation with clinicopathological features and prognosis in colorectal cancer patients. Int. J. Biol. Markers 30, e88–e95 (2015).
Jardim, D. L., Wheler, J. J., Hess, K., Tsimberidou, A. M., Zinner, R., Janku, F. et al. FBXW7 mutations in patients with advanced cancers: clinical and molecular characteristics and outcomes with mTOR inhibitors. PLoS ONE 9, e89388 (2014).
Acknowledgements
We wish to thank the OMITERC consortium and the Fondazione Sandro Pitigliani per la lotta contro i tumori ONLUS.
Author information
Authors and Affiliations
Contributions
F.S.: single CTC isolation, mutational analysis of CTCs and cfDNA, data analysis and manuscript writing; S.G.: management of clinical samples, data analysis and manuscript writing; I.M.: NGS data analysis; F.G. and F.D.L.: CTC counting by CellSearch; S.P.: clinical data management and critical revision of the manuscript; E.G. and M.B.: patients’ enrolment, clinical data collection and analysis; F.C. and L.M. mutational analysis of tumour tissues; M.P. and F.D.C.: conceptualisation, funding acquisition and critical revision of manuscript; P.P.: study design, project supervision and manuscript writing; L.A.: patients’ enrolment, clinical data management and study design.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Local Ethical Committee (Comitato Etico di Area Vasta Centro AOU Careggi, Firenze, Italy), reference number CEAVC BIO 16.028_10033_bio. Written informed consent to participate was obtained from all patients. The study was performed in accordance with the Declaration of Helsinki.
Consent to publish
Not applicable.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Funding information
This research was funded by Regione Toscana FAS 2007-2013, call FAS salute 2014, OMITERC project, grant 4421.02102014.072000037.
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Salvianti, F., Gelmini, S., Mancini, I. et al. Circulating tumour cells and cell-free DNA as a prognostic factor in metastatic colorectal cancer: the OMITERC prospective study. Br J Cancer 125, 94–100 (2021). https://doi.org/10.1038/s41416-021-01399-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01399-6
This article is cited by
-
Circulating tumour cells in gastrointestinal cancers: food for thought?
British Journal of Cancer (2023)
-
The non-invasive diagnosis of colorectal cancer via a SOX9-based gene panel
Clinical and Experimental Medicine (2023)
-
Integrating circulating-free DNA (cfDNA) analysis into clinical practice: opportunities and challenges
British Journal of Cancer (2022)
-
Circulating tumor DNA profiling for childhood brain tumors: Technical challenges and evidence for utility
Laboratory Investigation (2022)
-
Profiling circulating tumour cells and cell free DNA together in metastatic colon cancer
British Journal of Cancer (2021)