Abstract
Background
Chemoresistance is one of the major factors for treatment failure in OSCC. Identifying key resistance triggering molecules will be useful strategy for developing novel treatment methods.
Methods
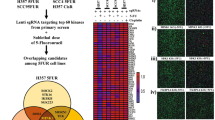
To identify the causative factors of chemoresistance, we performed RNA sequencing and global proteomic profiling of human OSCC lines presenting with sensitive, early and late cisplatin-resistance patterns.
Results
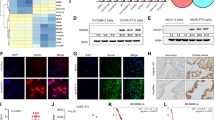
From the common set of dysregulated genes from both the analysis, RRBP1 was identified to be upregulated in both early and late cisplatin-resistant cells with respect to the sensitive counterpart. Analysis of OSCC patient sample indicates that RRBP1 expression is upregulated in chemotherapy-non-responder tumours as compared to chemotherapy-responder tumours. Genetic (knockout) or pharmacological (Radezolid, represses expression of RRBP1) inhibition of RRBP1 restores cisplatin-mediated cell death in chemo-resistant OSCC. Mechanistically, RRBP1 regulates Yes-associated protein1 (YAP1), a key protein in the Hippo pathway to induce chemoresistance. The PDC xenograft data suggests that knockout of RRBP1 induces cisplatin-mediated cell death and facilitates a significant reduction of tumour burden.
Conclusion
Overall, our data suggests that (I) RRBP1 is a major driver of cisplatin-resistance in OSCC, (II) RRBP1 regulates YAP1 expression to mediate cisplatin-resistance, (III) Radezolid represses RRBP1 expression and (IV) targeting RRBP1 reverses cisplatin-induced chemoresistance in advanced OSCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
07 May 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41416-021-01414-w
References
Dikshit, R., Gupta, P. C., Ramasundarahettige, C., Gajalakshmi, V., Aleksandrowicz, L., Badwe, R. et al. Cancer mortality in India: a nationally representative survey. Lancet 379, 1807–1816 (2012).
Huang, S. H. & O’Sullivan, B. Oral cancer: current role of radiotherapy and chemotherapy. Med. Oral. Patol. Oral. Cir. Bucal 18, e233–e240 (2013).
Ackerstaff, A. H., Rasch, C. R., Balm, A. J., de Boer, J. P., Wiggenraad, R., Rietveld, D. H. et al. Five-year quality of life results of the randomized clinical phase III (RADPLAT) trial, comparing concomitant intra-arterial versus intravenous chemoradiotherapy in locally advanced head and neck cancer. Head Neck 34, 974–980 (2012).
Wang, C., Liu, X. Q., Hou, J. S., Wang, J. N. & Huang, H. Z. Molecular mechanisms of chemoresistance in oral cancer. Chin. J. Dent. Res. 19, 25–33 (2016).
Vishak, S., Rangarajan, B. & Kekatpure, V. D. Neoadjuvant chemotherapy in oral cancers: selecting the right patients. Indian J. Med. Paediatr. Oncol. 36, 148–153 (2015).
Maji, S., Panda, S., Samal, S. K., Shriwas, O., Rath, R., Pellecchia, M. et al. Bcl-2 antiapoptotic family proteins and chemoresistance in cancer. Adv. Cancer Res. 137, 37–75 (2018).
Wanker, E. E., Sun, Y., Savitz, A. J. & Meyer, D. I. Functional characterization of the 180-kD ribosome receptor in vivo. J. Cell Biol. 130, 29–39 (1995).
Savitz, A. J. & Meyer, D. I. 180-kD ribosome receptor is essential for both ribosome binding and protein translocation. J. Cell Biol. 120, 853–863 (1993).
Ueno, T., Tanaka, K., Kaneko, K., Taga, Y., Sata, T., Irie, S. et al. Enhancement of procollagen biosynthesis by p180 through augmented ribosome association on the endoplasmic reticulum in response to stimulated secretion. J. Biol. Chem. 285, 29941–29950 (2010).
Maji, S., Shriwas, O., Samal, S. K., Priyadarshini, M., Rath, R., Panda, S. et al. STAT3- and GSK3beta-mediated Mcl-1 regulation modulates TPF resistance in oral squamous cell carcinoma. Carcinogenesis 40, 173–183 (2019).
Samal, S. K., Routray, S., Veeramachaneni, G. K., Dash, R. & Botlagunta, M. Ketorolac salt is a newly discovered DDX3 inhibitor to treat oral cancer. Sci. Rep. 5, 9982 (2015).
Arya, R., Dabral, D., Faruquee, H. M., Mazumdar, H., Patgiri, S. J., Deka, T. et al. Serum small extracellular vesicles proteome of tuberculosis patients demonstrated deregulated immune response. Proteom. Clin. Appl. 14, e1900062 (2020).
Shriwas, O., Priyadarshini, M., Samal, S. K., Rath, R., Panda, S., Das Majumdar, S. K. et al. DDX3 modulates cisplatin resistance in OSCC through ALKBH5-mediated m(6)A-demethylation of FOXM1 and NANOG. Apoptosis https://doi.org/10.1007/s10495-020-01591-8 (2020).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Hung, V., Lam, S. S., Udeshi, N. D., Svinkina, T., Guzman, G., Mootha, V. K. et al. Proteomic mapping of cytosol-facing outer mitochondrial and ER membranes in living human cells by proximity biotinylation. elife https://doi.org/10.7554/eLife.24463 (2017).
Zhao, B., Wei, X., Li, W., Udan, R. S., Yang, Q., Kim, J. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 (2007).
Kijima, T., Nakagawa, H., Shimonosono, M., Chandramouleeswaran, P. M., Hara, T., Sahu, V. et al. Three-dimensional organoids reveal therapy resistance of esophageal and oropharyngeal squamous cell carcinoma cells. Cell Mol. Gastroenterol. Hepatol. 7, 73–91 (2019).
Maji, S., Samal, S. K., Pattanaik, L., Panda, S., Quinn, B. A., Das, S. K. et al. Mcl-1 is an important therapeutic target for oral squamous cell carcinomas. Oncotarget 6, 16623–16637 (2015).
Tang, Z., Kang, B., Li, C., Chen, T. & Zhang, Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 47, W556–W560 (2019).
Rozengurt, E., Sinnett-Smith, J. & Eibl, G. Yes-associated protein (YAP) in pancreatic cancer: at the epicenter of a targetable signaling network associated with patient survival. Signal Transduct. Target Ther. 3, 11 (2018).
McDermott, M., Eustace, A. J., Busschots, S., Breen, L., Crown, J., Clynes, M. et al. In vitro development of chemotherapy and targeted therapy drug-resistant cancer cell lines: a practical guide with case studies. Front. Oncol. 4, 40 (2014).
Stordal, B. K., Davey, M. W. & Davey, R. A. Oxaliplatin induces drug resistance more rapidly than cisplatin in H69 small cell lung cancer cells. Cancer Chemother. Pharmacol. 58, 256–265 (2006).
Ma, J., Maliepaard, M., Kolker, H. J., Verweij, J. & Schellens, J. H. Abrogated energy-dependent uptake of cisplatin in a cisplatin-resistant subline of the human ovarian cancer cell line IGROV-1. Cancer Chemother. Pharmacol. 41, 186–192 (1998).
Shen, D. W., Akiyama, S., Schoenlein, P., Pastan, I. & Gottesman, M. M. Characterisation of high-level cisplatin-resistant cell lines established from a human hepatoma cell line and human KB adenocarcinoma cells: cross-resistance and protein changes. Br. J. Cancer 71, 676–683 (1995).
Liang, X. J., Shen, D. W., Garfield, S. & Gottesman, M. M. Mislocalization of membrane proteins associated with multidrug resistance in cisplatin-resistant cancer cell lines. Cancer Res. 63, 5909–5916 (2003).
Tsai, H. Y., Yang, Y. F., Wu, A. T., Yang, C. J., Liu, Y. P., Jan, Y. H. et al. Endoplasmic reticulum ribosome-binding protein 1 (RRBP1) overexpression is frequently found in lung cancer patients and alleviates intracellular stress-induced apoptosis through the enhancement of GRP78. Oncogene 32, 4921–4931 (2013).
Telikicherla, D., Marimuthu, A., Kashyap, M. K., Ramachandra, Y. L., Mohan, S., Roa, J. C. et al. Overexpression of ribosome binding protein 1 (RRBP1) in breast cancer. Clin. Proteomics 9, 7 (2012).
Pan, Y., Cao, F., Guo, A., Chang, W., Chen, X., Ma, W. et al. Endoplasmic reticulum ribosome-binding protein 1, RRBP1, promotes progression of colorectal cancer and predicts an unfavourable prognosis. Br. J. Cancer 113, 763–772 (2015).
Wang, L., Wang, M., Zhang, M., Li, X., Zhu, Z. & Wang, H. Expression and significance of RRBP1 in esophageal carcinoma. Cancer Manag. Res. 10, 1243–1249 (2018).
Liu, S., Lin, M., Ji, H., Ding, J., Zhu, J., Ma, R. et al. RRBP1 overexpression is associated with progression and prognosis in endometrial endometrioid adenocarcinoma. Diagn. Pathol. 14, 7 (2019).
Li, T., Wang, Q., Hong, X., Li, H., Yang, K., Li, J. et al. RRBP1 is highly expressed in prostate cancer and correlates with prognosis. Cancer Manag. Res. 11, 3021–3027 (2019).
Ma, J., Ren, S., Ding, J., Liu, S., Zhu, J., Ma, R. et al. Expression of RRBP1 in epithelial ovarian cancer and its clinical significance. Biosci. Rep. https://doi.org/10.1042/BSR20190656 (2019).
Han, Y. Analysis of the role of the Hippo pathway in cancer. J. Transl. Med. 17, 116 (2019).
Misra, J. R. & Irvine, K. D. The Hippo signaling network and its biological functions. Annu. Rev. Genet. 52, 65–87 (2018).
Gujral, T. S. & Kirschner, M. W. Hippo pathway mediates resistance to cytotoxic drugs. Proc. Natl Acad. Sci. USA 114, E3729–E3738 (2017).
Acknowledgements
O.S. is a UGC-SRF, S.M. is UGC-JRF, P.M. is CSIR SRF. S.R.K. and F.P. received fellowship from Department of Biotechnology. K.C.M. received institutional fellowship.
Author information
Authors and Affiliations
Contributions
O.S., S.M., R.A., P.M., S.R.K., F.P., S.K., P.P. and K.C.M. performed experiments, and analysed the data, under the direction of R.D. and R.K.N. R.R., D.K.M., A.D. and S.K.M. performed part of experiments. O.S. and R.D. designed experiments and supervised the study. O.S., R.D. and R.K.N. wrote the manuscript. All authors approved the final version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by Human Ethics committees (HEC) of Institute of Life Sciences, (84/HEC/18) and All India Institute of Medical Sciences (AIIMS), (T/EMF/Surg.Onco/19/03). The animal-related experiments were approved by Institutional Animal Ethics Committee of Institute of Life Sciences, (ILS/IAEC-147-AH/FEB-19).
Data availability
All the mass spectrometry data files (. raw and. mgf) with result files were deposited in the ProteomeXchange Consortium (PXD0016977) and all the RNA-seq data file were deposited in ArrayExpress (E-MTAB-9697).
Competing interests
The authors declare no competing interests.
Funding information
Grant support: This work is supported by Institute of Life Sciences, Bhubaneswar intramural support, ICMR (5/13/9/2019-NCD-III) and DBT BT/INF/22/SP28293/2018 (for imaging facility). Core support from International Centre for Genetic Engineering and Biotechnology to R.K.N. is highly acknowledged.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: During the proof process the authors unfortunately removed of one the co-corresponding authors. The correct version should read: Correspondence: Rupesh Dash (rupesh.dash@gmail.com) or Ranjan K. Nanda (HYPERLINK “mailto:ranjan@icgeb.res.in” ranjan@icgeb.res.in).
Supplementary information
41416_2021_1336_MOESM3_ESM.tif
Supplementary figure 3: The confirmation of RRBP1 KO in OSCC Chemoresistance cell line done by using GeneArt®Genomic Cleavage Detection (GCD) Kit (Life technology) with PCR primers listed in supplem
Rights and permissions
About this article
Cite this article
Shriwas, O., Arya, R., Mohanty, S. et al. RRBP1 rewires cisplatin resistance in oral squamous cell carcinoma by regulating Hippo pathway. Br J Cancer 124, 2004–2016 (2021). https://doi.org/10.1038/s41416-021-01336-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01336-7
This article is cited by
-
Genomic alterations in oral multiple primary cancers
International Journal of Oral Science (2024)