Abstract
Background
Eribulin is a microtubule-targeting agent approved for the treatment of advanced or metastatic breast cancer (BC) previously treated with anthracycline- and taxane-based regimens. PIK3CA mutation is associated with worse response to chemotherapy in oestrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) metastatic BC. We aimed to evaluate the role of phosphoinositide 3-kinase (PI3K)/AKT pathway mutations in eribulin resistance.
Methods
Resistance to eribulin was evaluated in HER2− BC cell lines and patient-derived tumour xenografts, and correlated with a mutation in the PI3K/AKT pathway.
Results
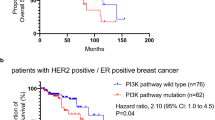
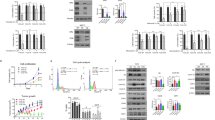
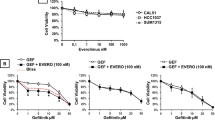
Eleven out of 23 HER2− BC xenografts treated with eribulin exhibited disease progression. No correlation with ER status was detected. Among the resistant models, 64% carried mutations in PIK3CA, PIK3R1 or AKT1, but only 17% among the sensitive xenografts (P = 0.036). We observed that eribulin treatment induced AKT phosphorylation in vitro and in patient tumours. In agreement, the addition of PI3K inhibitors reversed primary and acquired resistance to eribulin in xenograft models, regardless of the genetic alterations in PI3K/AKT pathway or ER status. Mechanistically, PI3K blockade reduced p21 levels likely enabling apoptosis, thus sensitising to eribulin treatment.
Conclusions
PI3K pathway activation induces primary resistance or early adaptation to eribulin, supporting the combination of PI3K inhibitors and eribulin for the treatment of HER2− BC patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cardoso, F., Spence, D., Mertz, S., Corneliussen-James, D., Sabelko, K., Gralow, J. et al. Global analysis of advanced/metastatic breast cancer: Decade report (2005–2015). Breast 39, 131–138 (2018).
Cardoso, F., Senkus, E., Costa, A., Papadopoulos, E., Aapro, M., André, F. et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann. Oncol. 29, 1634–1657 (2018).
Balic, M., Thomssen, C., Würstlein, R., Gnant, M. & Harbeck, N. St. Gallen/Vienna 2019: a brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care. 14, 103–110 (2019).
Cortes, J., O’Shaughnessy, J., Loesch, D., Blum, J. L., Vahdat, L. T., Petrakova, K. et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet 377, 914–923 (2011).
McIntyre, K., O’Shaughnessy, J., Schwartzberg, L., Glück, S., Berrak, E., Song, J. X. et al. Phase 2 study of eribulin mesylate as first-line therapy for locally recurrent or metastatic human epidermal growth factor receptor 2-negative breast cancer. Breast Cancer Res Treat. 146, 321–328 (2014).
Wong, K.-K., Engelman, J. A. & Cantley, L. C. Targeting the PI3K pathway in cancer. Curr. Opin. Genet Dev. 8, 627–644 (2009).
Coqueret, O. New roles for p21 and p27 cell-cycle inhibitors: A function for each cell compartment? Trends Cell Biol. 13, 65–70 (2003).
Sherr, C. J. & Roberts, J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 (1999).
Blain, S. W. Switching cyclin D-Cdk4 kinase activity on and off. Cell Cycle 7, 892–898 (2008).
Labaer, J., Garrett, M. D., Stevenson, L. F., Slingerland, J. M., Sandhu, C., Chou, H. S. et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11, 847–862 (1997).
Reynisdóttir, I. & Massagué, J. The subcellular locations of pl5(Ink4b) and p27(Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 11, 492–503 (1997).
Harper, J. W., Elledge, S. J., Keyomarsi, K., Dynlacht, B., Tsai, L. H., Zhang, P. et al. Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell 6, 387–p400 (1995).
Suzuki, A., Kawano, H., Hayashida, M., Hayasaki, Y., Tsutomi, Y. & Akahane, K. Procaspase 3/p21 complex formation to resist Fas-mediated cell death is initiated as a result of the phosphorylation of p21 by protein kinase A. Cell Death Differ. 7, 721–728 (2000).
Stemke-Hale, K., Gonzalez-Angulo, A. M., Lluch, A., Neve, R. M., Kuo, W. L., Davies, M. et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 68, 6084–6091 (2008).
López-Knowles, E., O’Toole, S. A., McNeil, C. M., Millar, E. K. A., Qiu, M. R., Crea, P. et al. PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. Int. J. Cancer 126, 1121–1131 (2010).
Bareche, Y., Venet, D., Ignatiadis, M., Aftimos, P., Piccart, M., Rothe, F. et al. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann. Oncol. 29, 895–902 (2018).
Koboldt, D. C., Fulton, R. S., McLellan, M. D., Schmidt, H., Kalicki-Veizer, J., McMichael, J. F. et al. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Huang, W. C. & Hung, M. C. Induction of Akt activity by chemotherapy confers acquired resistance. J. Formos. Med. Assoc. 108, 180–194 (2009).
Wallin, J. J., Guan, J., Prior, W. W., Edgar, K. A., Kassees, R., Sampath, D. et al. Nuclear phospho-Akt increase predicts synergy of PI3K inhibition and doxorubicin in breast and ovarian cancer. Sci. Transl. Med. 2, 1–9 (2010).
Clark, A. S., West, K., Streicher, S., Dennis, P. A., Brueggemeier, R. W., Shapiro, C. L. et al. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol. Cancer Ther. 1, 707–717 (2002).
Rodon, J., Dienstmann, R., Serra, V. & Tabernero, J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat. Rev. Clin. Oncol. 10, 143–153 (2013).
Martín, M., Chan, A., Dirix, L., O’Shaughnessy, J., Hegg, R., Manikhas, A. et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4). Ann. Oncol. 28, 313–320 (2017).
Isakoff, S. J., Engelman, J. A., Irie, H. Y., Luo, J., Brachmann, S. M., Pearline, R. V. et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 65, 10992–11000 (2005).
VanderWeele, D. J., Zhou, R. & Rudin, C. M. Akt up-regulation increases resistance to microtubule-directed chemotherapeutic agents through mammalian target of rapamycin. Mol. Cancer Ther. 3, 1605–1613 (2004).
Fujiwara, Y., Hosokawa, Y., Watanabe, K., Tanimura, S., Ozaki, K. -I. & Kohno, M. Blockade of the phosphatidylinositol-3-kinase-Akt signaling pathway enhances the induction of apoptosis by microtubule-destabilizing agents in tumor cells in which the pathway is constitutively activated. Mol. Cancer Ther. 6, 1133–1142 (2007).
Wallin, J. J., Guan, J., Prior, W. W., Lee, L. B., Berry, L., Belmont, L. D. et al. GDC-0941, a novel class I selective PI3K inhibitor, enhances the efficacy of docetaxel in human breast cancer models by increasing cell death in vitro and in vivo. Clin. Cancer Res. 18, 3901–3911 (2012).
Hirai, H., Sootome, H., Nakatsuru, Y., Miyama, K., Taguchi, S., Tsujioka, K. et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol. Cancer Ther. 9, 1956–1967 (2010).
McDonald, G. T., Sullivan, R., Paré, G. C. & Graham, C. H. Inhibition of phosphatidylinositol 3-kinase promotes tumor cell resistance to chemotherapeutic agents via a mechanism involving delay in cell cycle progression. Exp. Cell Res. 316, 3197–3206 (2010).
Rajput, S., Guo, Z., Li, S. & Ma, C. X. PI3K inhibition enhances the anti-tumor effect of eribulin in triple negative breast cancer. Oncotarget 10, 3667–3680 (2019).
Owusu-Brackett, N., Kenerson, H. L., Riggle, K. M., Turnham, R., Sullivan, K., Bauer, R. et al. TAK228 enhances antitumor activity of eribulin in triple negative breast cancer. Oncotarget 10, 5011–5019 (2019).
Wen, W., Marcinkowski, E., Luyimbazi, D., Luu, T., Xing, Q., Yan, J. et al. Eribulin synergistically increases anti-tumor activity of an mTOR inhibitor by inhibiting pAKT/pS6K/pS6 in triple negative breast. Cancer Cells 8, 1–15 (2019).
Beaufils, F., Cmiljanovic, N., Cmiljanovic, V., Bohnacker, T., Melone, A., Marone, R. et al. 5-(4,6-Dimorpholino-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine (PQR309), a potent, brain-penetrant, orally bioavailable, pan-class i PI3K/mTOR inhibitor as clinical candidate in oncology. J. Med. Chem. 60, 7524–7538 (2017).
Gong, J. P., Traganos, F. & Darzynkiewicz, Z. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal. Biochem. 218, 314–319 (1994).
Krishan, A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J. Cell Biol. 66, 188–193 (1975).
Bruna, A., Rueda, O. M., Greenwood, W., Batra, A. S., Callari, M., Batra, R. N. et al. A Biobank of breast cancer explants with preserved intra-tumor heterogeneity to screen anticancer compounds. Cell 167, 260–274.e22 (2016).
Tentler, J. J., Tan, A. C., Weekes, C. D., Jimeno, A., Leong, S., Pitts, T. M. et al. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 9, 338–350 (2012).
Festing, M. F. W. Design and statistical methods in studies using animal models of development. ILAR J. 47, 5–14 (2006).
Gao, H., Korn, J. M., Ferretti, S., Monahan, J. E., Wang, Y., Singh, M. et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med 21, 1318–1325 (2015).
Therasse, P., Arbuck, S. G., Eisenhauer, E. A., Wanders, J., Kaplan, R. S., Rubinstein, L. et al. New guidelines to evaluate the response to treatment in solid tumors. J. Natl Cancer Inst. 92, 205–216 (2000).
Cheng, D. T., Mitchell, T. N., Zehir, A., Shah, R. H., Benayed, R., Syed, A. et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J. Mol. Diagn. 17, 251–264 (2015).
Juric, D., Castel, P., Griffith, M., Griffith, O. L., Won, H. H., Ellis, H. et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature 518, 240–244 (2015).
Wee, S., Wiederschain, D., Maira, S.-M., Loo, A., Miller, C., DeBeaumont, R. et al. PTEN-deficient cancers depend on PIK3CB. Proc. Natl Acad. Sci. USA 105, 13057–13062 (2008).
Jordan, M. A. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol. Cancer Ther. 4, 1086–1095 (2005).
Kalinsky, K., Jacks, L. M., Heguy, A., Patil, S., Drobnjak, M., Bhanot, U. K. et al. PIK3CA mutation associates with improved outcome in breast cancer. Clin. Cancer Res. 15, 5049–5059 (2009).
Zardavas, D., Te Marvelde, L., Milne, R. L., Fumagalli, D., Fountzilas, G., Kotoula, V. et al. Tumor PIK3CA genotype and prognosis in early-stage breast cancer: a pooled analysis of individual patient data. J. Clin. Oncol. 36, 981–990 (2018).
Mosele, F., Stefanovska, B., Lusque, A., Tran Dien, A., Garberis, I., Droin, N. et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann. Oncol. 31, 377–386 (2020).
Yuan, H., Chen, J., Liu, Y., Ouyang, T., Li, J., Wang, T. et al. Association of PIK3CA mutation status before and after neoadjuvant chemotherapy with response to chemotherapy in women with breast cancer. Clin. Cancer Res. 21, 4365–4372 (2015).
Murtaza, M., Dawson, S. J., Tsui, D. W. Y., Gale, D., Forshew, T., Piskorz, A. M. et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 497, 108–112 (2013).
Brachmann, S. M., Kleylein-Sohn, J., Gaulis, S., Kauffmann, A., Blommers, M. J. J., Kazic-Legueux, M. et al. Characterization of the mechanism of action of the pan class I PI3K inhibitor NVP-BKM120 across a broad range of concentrations. Mol. Cancer Ther. 11, 1747–1757 (2012).
Lee, J. S., Yost, S. E., Blanchard, S., Schmolze, D., Yin, H. H., Pillai, R. et al. Phase I clinical trial of the combination of eribulin and everolimus in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 21, 1–13 (2019).
Turner, N. C., Alarcón, E., Armstrong, A. C., Philco, M., López Chuken, Y. A., Sablin, M.-P. et al. BEECH: a dose-finding run-in followed by a randomised phase II study assessing the efficacy of AKT inhibitor capivasertib (AZD5363) combined with paclitaxel in patients with estrogen receptor-positive advanced or metastatic breast cancer, and in a PIK3CA. Ann. Oncol. 30, 774–780 (2019).
Schmid, P., Abraham, J., Chan, S., Wheatley, D., Brunt, M., Nemsadze, G. et al. AZD5363 plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (PAKT): a randomised, double-blind, placebo-controlled, phase II trial. J. Clin. Oncol. 36(Suppl.), 1007–1007 (2018).
Kim, S.-B., Dent, R., Im, S.-A., Espié, M., Blau, S., Tan, A. R. et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 18, 1360–1372 (2017).
Oliveira, M., Saura, C., Nuciforo, P., Calvo, I., Andersen, J. & Gil, M. FAIRLANE, a double-blind placebo-controlled randomized phase II trial of neoadjuvant ipatasertib plus paclitaxel for early triple-negative breast cancer. Ann. Oncol. 30, 1289–1297 (2019).
Bosch, A., Li, Z., Bergamaschi, A., Ellis, H., Toska, E., Prat, A. et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci. Transl. Med. 7, 283ra51 (2015).
Toska, E., Osmanbeyoglu, H. U., Castel, P., Chan, C., Hendrickson, R. C., Elkabets, M. et al. PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science 335, 1324–1330 (2017).
Jain, S. & Vahdat, L. T. Eribulin mesylate. Clin. Cancer Res. 17, 6615–6622 (2011).
Agoulnik, S., Kuznetsov, G., Tendyke, K., Parent, L. A., Marsh, J. P., Twine, N. et al. Sensitivity to halichondrin analog E7389 and hemiasterlin analog E7974 correlates with βIII tubulin isotype expression in human breast cancer cell lines. J. Clin. Oncol. 23(Suppl.), 2012–2012 (2017).
Mabuchi, S., Ohmichi, M., Kimura, A., Hisamoto, K., Hayakawa, J., Nishio, Y. et al. Inhibition of phosphorylation of BAD and Raf-1 by Akt sensitizes human ovarian cancer cells to paclitaxel. J. Biol. Chem. 277, 33490–33500 (2002).
Hayasaka, N., Takada, K., Nakamura, H., Arihara, Y., Kawano, Y., Osuga, T. et al. Combination of eribulin plus AKT inhibitor evokes synergistic cytotoxicity in soft tissue sarcoma cells. Sci. Rep. 9, 1–8 (2019).
Bozulic, L., Surucu, B., Hynx, D. & Hemmings, B. A. PKBα/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol. Cell 30, 203–213 (2008).
Mitsuuchi, Y., Johnson, S. W., Selvakumaran, M., Williams, S. J., Hamilton, T. C. & Testa, J. R. The phosphatidylinositol 3-kinase/AKT signal transduction pathway plays a critical role in the expression of p21(WAF1/CIP1/SDI1) induced by cisplatin and paclitaxel. Cancer Res. 60, 5390–5394 (2000).
Charrier-Savournin, F. B., Château, M. T., Gire, V., Sedivy, J., Piette, J. & Dulić, V. p21-mediated nuclear retention of cyclin B1-Cdk1 in response to genotoxic stress. Mol. Biol. Cell 15, 3965–3976 (2004).
Acknowledgements
We are grateful to all the patients who kindly consented to the use of their tumours to develop this study and to all the personnel involved in sample collection from the Breast Surgical Unit, Breast Cancer Centre, and Department of Radiology Vall d’Hebron University Hospital and Molecular Oncology Group at Vall d’Hebron Institute of Oncology (VHIO). We also thank the Cellex Foundation for providing research facilities and equipment, as well as Ana Vivancos and the Cancer Genomics Group at VHIO and Joanne Soong and the Centre for Molecular Oncology at the MSKCC for providing technical and analytical support with the patient and PDX sequencing.
Author information
Authors and Affiliations
Contributions
A.G.-O., J.C. and V.S. designed the experiments and wrote the manuscript. A.G.-O., Y.H.I, M.A.R., C.G.-G., M.S.-G., F.R.-P., C.V., J.M.P.-G., A.L.-C., J.G., M.P., M.G., O.R., P.A., P.C., M.T.C., A.B., J.A., C.C., R.D., P.N., M.O., J.C. and V.S. helped in the acquisition of data and/or provided technical support (provided animals, acquired and managed patients, provided facilities, etc.). A.G.-O., Y.H.I., M.A.R., C.G.-G., M.S.-G., F.R.-P., C.V., R.D., M.O., J.C. and V.S. contributed in the analysis of data (e.g. statistical analysis, biostatistics, computational analysis and construction of databases).
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Fresh tumour samples from patients with breast cancer were collected following an Institutional Research Board-approved protocol and the associated written informed consent. The study was compliant with the Declaration of Helsinki.
Experiments were conducted following the European Union’s animal care directive (2010/63/EU) and were approved by the Ethical Committee of Animal Experimentation of the Vall d’Hebron Research Institute, the Catalan Government or by the National Research Ethics Service, Cambridgeshire (ref. 35 and https://caldaslab.cruk.cam.ac.uk/bcape/).
Data availability
All data generated or analysed during this study are included in this published article [and its Supplementary information files].
Competing interests
V.S. declares non-commercial research agreements with Genentech and Novartis. J.C. reports consulting for Roche, Celgene, Cellestia, AstraZeneca, Biothera Pharmaceutical, Merus, Seattle Genetics, Daiichi Sankyo, Erytech, Athenex, Polyphor, Lilly, Servier, Merck Sharp&Dohme, GSK, Leuko, Bioasis, Clovis Oncology and Boehringer Ingelheim; honoraria for Roche, Novartis, Celgene, Eisai, Pfizer, Samsung Bioepis, Lilly, Merck Sharp&Dohme and Daiichi Sankyo; research funding to the Institution: Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer Healthcare, Eisai, F. Hoffman-La Roche, Guardanth Health, Merck Sharp&Dohme, Pfizer, Piqur Therapeutics, Puma C and Queen Mary University of London; stock, patents and intellectual property of MedSIR; and travel, accommodation and other expenses for Roche, Novartis, Eisai, Pfizer and Daiichi Sankyo. M.O. declares research support from AstraZeneca, Genentech and Philips Healthcare; consultant role for Roche, GSK, PUMA Biotechnology and AstraZeneca; and has received honoraria from Roche. M.S.-G. is on the scientific advisory board of Menarini Ricerche and the Bioscience Institute, has received research funds from Puma Biotechnology, Daiichi-Sankio, Targimmune, Immunomedics and Menarini Ricerche, and is a cofounder of Medendi.org. C.C. is a member of AstraZeneca’s External Science Panel, of Illumina’s Scientific Advisory Board and is a recipient of research grants (administered by the University of Cambridge) from AstraZeneca, Genentech, Roche and Servier. R.D. is on advisory role of AstraZeneca, Roche and Boehringer-Ingelheim and has received speaker’s fees from Roche, Symphogen, IPSEN, Amgen, Servier, Sanofi and MSD; and research support from Merck. J.M.P.-G. reports an advisory role with Roche and Lilly. A.L.-C. has been a consultant for Roche, GlaxoSmithKline, Novartis, Celgene, Eisai and AstraZeneca in the previous 12 months and has stock options for Medica Scientia Innovation Research SL (MedSIR). All other authors declare no competing interests.
Funding information
We acknowledge the GHD-Pink programme, the FERO Foundation and the Orozco Family for supporting this study [to V.S.], as well as Fundación Mútua Madrileña [to J.C.]. This study has also been supported by the Catalan Agency AGAUR [2017 SGR 540 to V.S.]. V.S. is supported by the Miguel Servet Programme (ISCIII) [CPII19/00033]. A.G.-O. was awarded with a fellowship from the Agència de Gestió d’Ajuts Universitaris i de Recerca (FI-AGAUR, 2015 FI_B 01075), and M.S.-G. with a Marie Slodowska-Curie Innovative Training Networks (MSCA-ITN) Ph.D. fellowship (H2020-MSCA-ITN-2015_675392). The xenograft programme in the Caldas Laboratory was supported by Cancer Research UK and also received funding from an EU H2020 Network of Excellence (EuroCAN).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gris-Oliver, A., Ibrahim, Y.H., Rivas, M.A. et al. PI3K activation promotes resistance to eribulin in HER2-negative breast cancer. Br J Cancer 124, 1581–1591 (2021). https://doi.org/10.1038/s41416-021-01293-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01293-1
This article is cited by
-
XENERA-1: a randomised double-blind Phase II trial of xentuzumab in combination with everolimus and exemestane versus everolimus and exemestane in patients with hormone receptor-positive/HER2-negative metastatic breast cancer and non-visceral disease
Breast Cancer Research (2023)
-
Eribulin activity in soft tissue sarcoma monolayer and three-dimensional cell line models: could the combination with other drugs improve its antitumoral effect?
Cancer Cell International (2021)