Abstract
Background
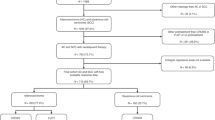
A high Mandard score implies a non-response to chemotherapy in oesophageal adenocarcinoma. However, some patients exhibit tumour volume reduction and a nodal response despite a high score. This study examines survival and recurrence patterns in these patients.
Methods
Clinicopathological factors were analysed using multivariable Cox regression assessing time to death and recurrence. Computed tomography-estimated tumour volume change was examined in a subgroup of consecutive patients.
Results
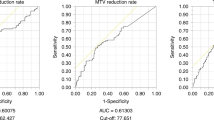
Five hundred and fifty-five patients were included. Median survival was 55 months (Mandard 1–3) and 21 months (Mandard 4 and 5). In the Mandard 4 and 5 group (332 patients), comparison between complete nodal responders and persistent nodal disease showed improved survival (90 vs 18 months), recurrence rates (locoregional 14.75 vs 28.74%, systemic 24.59 vs 48.42%) and circumferential resection margin positivity (22.95 vs 68.11%). Complete nodal response independently predicted improved survival (hazard ratio 0.34 (0.16–0.74). Post-chemotherapy tumour volume reduction was greater in patients with a complete nodal response (−16.3 vs −7.7 cm3, p = 0.033) with no significant difference between Mandard groups.
Conclusion
Patients with a complete nodal response to chemotherapy have significantly improved outcomes despite a poor Mandard score. High Mandard score does not correspond with a non-response to chemotherapy in all cases and patients with nodal downstaging may still benefit from adjuvant chemotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cunningham, D., Starling, N., Rao, S., Iveson, T., Nicolson, M., Coxon, F. et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 358, 36–46 (2008).
Allum, W. H., Stenning, S. P., Bancewicz, J., Clark, P. I. & Langley, R. E. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J. Clin. Oncol. 27, 5062–5067 (2009).
Al-Batran, S.-E., Homann, N., Schmalenberg, H., Kopp, H.-G., Haag, G. M., Luley, K. B. et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): a multicenter, randomized phase 3 trial. J. Clin. Oncol. 35(Suppl.), 4004–4004 (2017).
van Hagen, P., Hulshof, M., van Lanschot, J., Steyerberg, E., van Berge Henegouwen, M., Wijnhoven, B. et al. Preoperative chemoradiotherapy for esophageal or junctional cancer for the CROSS Group*. N. Engl. J. Med. 22366, 2074–2084 (2012).
Noble, F. Refining pathological evaluation of neoadjuvant therapy for adenocarcinoma of the esophagus. World J. Gastroenterol. 19, 9282 (2013).
Mandard, A.-M., Dalibard, F., Mandard, J.-C., Marnay, J., Henry-Amar, M., Petiot, J.-F. et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 73, 2680–2686 (1994).
zum Büschenfelde, C. M., Herrmann, K., Schuster, T., Geinitz, H., Langer, R., Becker, K. et al. (18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial. J. Nucl. Med. 52, 1189–1196 (2011).
Li, R., Tian-wu Chen, M., Hu, J., Guo, D., Xiao-ming Zhang, M., Deng, D. et al. Tumor volume of resectable adenocarcinoma of the esophagogastric junction at multidetector cT: association with regional lymph node metastasis and N Stage 1. Radiology 269, 130–138 (2013).
Tamandl, D., Gore, R. M., Fueger, B., Kinsperger, P., Hejna, M., Paireder, M. et al. Change in volume parameters induced by neoadjuvant chemotherapy provide accurate prediction of overall survival after resection in patients with oesophageal cancer. Eur. Radiol. 26, 311–321 (2016).
Davies, A. R., Myoteri, D., Zylstra, J., Baker, C. R., Wulaningsih, W., Van Hemelrijck, M. et al. Lymph node regression and survival following neoadjuvant chemotherapy in oesophageal adenocarcinoma. Br. J. Surg. 105, 1639–1649 (2018).
Mapstone, N. P. Dataset for the Histopathological Reporting of Oesophageal Carcinoma (The Royal College of Pathologists, 2007).
Rosset, A., Spadola, L. & Ratib, O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J. Digit. Imaging 17, 205–216 (2004).
Noble, F., Lloyd, M. A., Turkington, R., Griffiths, E., O’Donovan, M., O’Neill, J. R. et al. Multicentre cohort study to define and validate pathological assessment of response to neoadjuvant therapy in oesophagogastric adenocarcinoma. Br. J. Surg. 104, 1816–1828 (2017).
Findlay, J. M., Bradley, K. M., Wang, L. M., Franklin, J. M., Teoh, E. J., Gleeson, F. V. et al. Predicting pathologic response of esophageal cancer to neoadjuvant chemotherapy: the implications of metabolic nodal response for personalized therapy. J. Nucl. Med. 58, 266–275 (2017).
Yip, C., Cook, G. J. R., Landau, D. B., Davies, A. & Goh, V. Performance of different imaging modalities in assessment of response to neoadjuvant therapy in primary esophageal cancer. Dis. Esophagus 29, 116–130 (2016).
van Heijl, M., Phoa, S. S. K. S., van Berge Henegouwen, M. I., JMT, Omloo, Mearadji, B. M., Sloof, G. W. et al. Accuracy and reproducibility of 3D-CT measurements for early response assessment of chemoradiotherapy in patients with oesophageal cancer. Eur. J. Surg. Oncol. 37, 1064–1071 (2011).
Griffith, J. F., Chan, A. C., Chow, L. T., Leung, S. F., Lam, Y. H., Liang, E. Y. et al. Assessing chemotherapy response of squamous cell oesophageal carcinoma with spiral CT. Br. J. Radiol. 72, 678–684 (1999).
Konieczny, A., Meyer, P., Schnider, A., Komminoth, P., Schmid, M., Lombriser, N. et al. Accuracy of multidetector-row CT for restaging after neoadjuvant treatment in patients with oesophageal cancer. Eur. Radiol. 23, 2492–2502 (2013).
Weber, M.-A., Bender, K., von Gall, C. C., Stange, A., Grünberg, K., Ott, K. et al. Assessment of diffusion-weighted MRI and 18F-fluoro-deoxyglucose PET/CT in monitoring early response to neoadjuvant chemotherapy in adenocarcinoma of the esophagogastric junction. J. Gastrointestin. Liver Dis. 22, 45–52 (2013).
Malik, V., Lucey, J. A., Duffy, G. J., Wilson, L., McNamara, L., Keogan, M. et al. Early repeated 18F-FDG PET scans during neoadjuvant chemoradiation fail to predict histopathologic response or survival benefit in adenocarcinoma of the esophagus. J. Nucl. Med. 51, 1863–1869 (2010).
Gillham, C. M., Lucey, J. A., Keogan, M., Duffy, G. J., Malik, V., Raouf, A. A. et al. 18FDG uptake during induction chemoradiation for oesophageal cancer fails to predict histomorphological tumour response. Br. J. Cancer 95, 1174–1179 (2006).
Lordick, F., Ott, K., Krause, B.-J., Weber, W. A., Becker, K., Stein, H. J. et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 8, 797–805 (2007).
Tullie, L. G. C., Sohn, H.-M., Zylstra, J., Mattsson, F., Griffin, N., Sharma, N. et al. A role for tumor volume assessment in resectable esophageal cancer. Ann. Surg. Oncol. 23, 3063–3070 (2016).
Senthebane, D. A., Jonker, T., Rowe, A., Thomford, N. E., Munro, D., Dandara, C. et al. The role of tumor microenvironment in chemoresistance: 3D extracellular matrices as accomplices. Int. J. Mol. Sci. 19, 2861 (2018).
Siddik, Z. H. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22, 7265–7279 (2003).
Nakamura, K. & Smyth, M. J. Targeting cancer-related inflammation in the era of immunotherapy. Immunol. Cell Biol. 95, 325–332 (2017).
Jain, R. K. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 31, 2205–2218 (2013).
Lin, E. W., Karakasheva, T. A., Hicks, P. D., Bass, A. J. & Rustgi, A. K. The tumor microenvironment in esophageal cancer. Oncogene. Nat. Publ. Group 35, 5337–5349 (2016).
Makino, T., Yamasaki, M., Tanaka, K., Masuike, Y., Tatsumi, M., Motoori, M. et al. Metabolic tumor volume change predicts long-term survival and histological response to preoperative chemotherapy in locally advanced esophageal cancer. Ann. Surg. 270, 1090–1095 (2019).
Urakawa, S., Makino, T., Yamasaki, M., Tanaka, K., Miyazaki, Y., Takahashi, T. et al. Lymph node response to neoadjuvant chemotherapy as an independent prognostic factor in metastatic esophageal cancer. Ann Surg. https://doi.org/10.1097/SLA.0000000000003445 (2019).
Jones, S., Chen, W. D., Parmigiani, G., Diehl, F., Beerenwinkel, N., Antal, T. et al. Comparative lesion sequencing provides insights into tumor evolution. Proc. Natl Acad. Sci. USA 105, 4283–4288 (2008).
Zanoni, A., Verlato, G., Giacopuzzi, S., Motton, M., Casella, F., Weindelmayer, J. et al. ypN0: does it matter how you get there? Nodal downstaging in esophageal cancer. Ann. Surg. Oncol. 23, 998–1004 (2016).
Saunders, J. H., Bowman, C. R., Reece-Smith, A. M., Pang, V., Dorrington, M. S., Mumtaz, E. et al. The role of adjuvant platinum-based chemotherapy in esophagogastric cancer patients who received neoadjuvant chemotherapy prior to definitive surgery. J. Surg. Oncol. 115, 821–829 (2017).
Knight, W. R. C., Zylstra, J., Van Hemelrijck, M., Griffin, N., Jacques, A. E. T., Maisey, N. et al. Patterns of recurrence in oesophageal cancer following oesophagectomy in the era of neoadjuvant chemotherapy. BJS Open 1, 182–190 (2017).
On behalf of the Guy’s & St Thomas’ Oesophago-Gastric Research Group
O. Hynes1, G. Tham1, C. Iezzi1, R. Bott1, N. Maisey5, A. Gaya5, S. Ngan5, A. Qureshi5, M. Green6, A. Jacques2, V. Goh7, H. Deere8, F. Chang8, U. Mahadeva8, B. Gill-Barman8, S. George8, J. Dunn9, S. Zeki9 and J. Meenan9
Author information
Authors and Affiliations
Consortia
Contributions
Study concepts: W.R.C.K., C.R.B. and J.A.G. Study design: W.R.C.K., C.R.B., N.G., M.K., A.R.D. and J.A.G. Data acquisition: W.R.C.K., N.G. and A.R.D. Quality control of data and algorithms: W.R.C.K., W.W. and J.A.G. Data analysis and interpretation: W.R.C.K., W.W. and J.A.G. Statistical analysis: W.R.C.K., W.W. and J.A.G. Manuscript preparation: W.R.C.K. Manuscript editing: W.R.C.K., C.R.B., M.K., A.R.D. and J.A.G. Manuscript review: W.R.C.K, C.R.B., N.G., M.K., A.R.D., J.A.G. and W.W.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval for use of the database was obtained through the Integrated research application system (IRAS reference: 12-NW-0511). Appropriate consent was gained from all patients.
Consent to publish
Not applicable.
Data availability
Data were available on request from the authors.
Competing interests
The authors declare no competing interests.
Funding information
No funding was used for this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Knight, W.R.C., Baker, C.R., Griffin, N. et al. Does a high Mandard score really define a poor response to chemotherapy in oesophageal adenocarcinoma?. Br J Cancer 124, 1653–1660 (2021). https://doi.org/10.1038/s41416-021-01290-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01290-4