Abstract
Background
Radiogenomics is an emerging field that integrates “Radiomics” and “Genomics”. In the current study, we aimed to predict the genetic information of pancreatic tumours in a simple, inexpensive, and non-invasive manner, using cancer imaging analysis and radiogenomics. We focused on p53 mutations, which are highly implicated in pancreatic ductal adenocarcinoma (PDAC), and PD-L1, a biomarker for immune checkpoint inhibitor-based therapies.
Methods
Overall, 107 patients diagnosed with PDAC were retrospectively examined. The relationship between p53 mutations as well as PD-L1 abnormal expression and clinicopathological factors was investigated using immunohistochemistry. Imaging features (IFs) were extracted from CT scans and were used to create prediction models of p53 and PD-L1 status.
Results
We found that p53 and PD-L1 are significant independent prognostic factors (P = 0.008, 0.013, respectively). The area under the curve for p53 and PD-L1 predictive models was 0.795 and 0.683, respectively. Radiogenomics-predicted p53 mutations were significantly associated with poor prognosis (P = 0.015), whereas the predicted abnormal expression of PD-L1 was not significant (P = 0.096).
Conclusions
Radiogenomics could predict p53 mutations and in turn the prognosis of PDAC patients. Hence, prediction of genetic information using radiogenomic analysis may aid in the development of precision medicine.
Similar content being viewed by others
Background
Pancreatic cancer is an extremely lethal cancer, with poor prognosis and no established marker of survival. The overall 5-year survival rate is only 6%, and remains <25% even after curative surgery, thus making it one of the most lethal tumours.1 Recently, a whole-genome search was performed in pancreatic cancer, identifying four major genetic mutations, namely in KRAS, p53, CDKN2A and SMAD4/DPC4.2
In more than 90% of pancreatic cancers, mutation of KRAS has been observed. Currently, due to the high mutation rate of KRAS, it is reported that biopsy is performed to diagnose pancreatic cancer with pathological outcome and with mutated KRAS.3 p53, CDKN2A, and SMAD4 are tumour suppressor genes, and in pancreatic cancer, mutations have been observed in ~50–70% of p53 and in 30–50% of CDKN2A and SMAD4.2 In pancreatic cancer, p53 mutations are controversial, although they have been reported to correlate with worse prognosis.4
While the expression of programmed death ligand 1 (PD-L1) in tumour cells is considered to be a poor prognostic factor, it has attracted attention as a target and marker for anti-tumour drugs.5 There have been several reports of PD-L1-high expression groups being correlated with worse prognosis in pancreatic cancer.6 Immune checkpoint inhibitors (ICIs) that activate autoimmunity have shown effective results in lung cancer.7,8 They are expected to be effective in pancreatic cancer as well. Currently, there is growing expectation from precision medicine, which examines individual genetic information and uses it to analyse and select the optimal treatment for the particular patient. However, the study and availability of individualised treatments for each patient are limited by time and economy and would benefit to some extent from technical innovation in future.
Images from CT and MRI are originally qualitative data; however, they can be regarded as a matrix, since they are digital data as well. Therefore, they can be quantified using a mathematical method. Such quantitative values, namely image features (IF), can be extracted from CT and MRI data, and this research field is called radiomics. The field that integrates two different “omics” information—radiomics and genomics—is called radiogenomics.9 It researches for correlations between radiomics and genomics such as genomes and gene expression analysis.9
Radiogenomics is expected to predict the molecular profiles of tumours from image phenotypes easily, non-invasively, and inexpensively. In fact, some reports of radiogenomics have indeed predicted molecular profiles, which are clinically important in breast cancer, lung cancer, and glioblastoma, from image data.10,11,12 While there are reports predicting clinicopathological results from image data (Radiomics) in pancreatic cancer, there is no such report yet predicting genetic information like p53 and PD-L1 expression.13,14,15,16 The current study aimed to predict genetic information simply and inexpensively from images commonly used for cancer diagnosis and treatment. We evaluated p53 and PD-L1 expression by immunohistochemistry (IHC) and analysed their correlation with clinicopathological data, including prognosis. We examined whether the expression of p53 and PD-L1 could be predicted from CT images utilising the new field of radiogenomics.
Methods
Study population criteria
From January 2013 to December 2017, 140 patients were diagnosed with pancreatic cancer. Of those, 107 who did not receive preoperative chemotherapy, who followed postoperative clinical course, and who had a pathological diagnosis of pancreatic ductal adenocarcinoma (PDAC), were retrospectively examined. All patients provided written informed consent and the study was approved by our institutional ethics committee.
Immunohistochemistry of p53 and PD-L1
We measured p53 levels by IHC using mouse monoclonal anti-human p53 protein antibody (DO7; Nichirei Biosciences Inc., Tokyo, Japan) and rabbit monoclonal anti-human PD-L1 protein antibody (SP263; Ventana Medical Systems, Inc., Tucson, AZ, USA). Five-micron-thick sections were obtained from formalin-fixed, paraffin-embedded tissues and set aside for p53 antibody (DO7) and PD-L1 (SP263) assay using a VENTANA OptiView DAB universal kit (Roche, Bazel, Switzerland) and VENTANA BenchMark ULTRA automated slide stainer (Roche, Bazel, Switzerland). Heat-induced antigen retrieval was performed using Cell Conditioning 1 (CC1; Ventana Medical Systems) for 32 min at 100 °C, followed by application of the primary antibody against p53 for 16 min at 36 °C, that of CC1 for 64 min at 100 °C, and of the primary antibody against PD-L1 for 16 min at 36 °C.
The IHC results were scored based on the percent positivity of staining. Protein expression of p53 and PD-L1 was evaluated by two pathologists as the percentage of staining area of all tumour cells. p53 status was determined by the percentage range of stained tumour cell nuclei. PD-L1 status was determined by the percentage of tumour cells with membrane staining above background.
IHC scoring of p53, PD-L1 and variable definitions
In normal pancreatic tissues adjacent to the tumour, nuclear accumulation of p53 was observed in pancreatic ductal cells with scattered, non-specific weak nuclear staining using IHC (Fig. 1a); this was considered the negative control.4,17 p53 mutations resulted in either nuclear accumulation of p53 protein, which was defined as the overexpression type (Fig. 1b), or in the complete absence of p53, defined as the null type (Fig. 1c), in contrast to the negative control.4,17 Wild-type p53 was characterised by a staining pattern in tumour nuclei that was equivalent to the negative control (Fig. 1a). For statistical comparisons, cases with p53-stained nuclei of total tumour cells exceeding 20% or completely absent in tumour cells were defined as “p53-positive”. There was no uniform opinion about how to evaluate PD-L1 expression in IHC.6 According to previous reports, the threshold for staining percentage of tumour cells was set at 1–10%, and cases with PD-L1-stained cells >1% of the total tumour cells were considered as PD-L1-positive (Fig. 1d, e).6 An 80% agreement among the pathologists involved in immunostaining evaluation was set as the criterion. When pathologists disagreed with regard to an evaluation, a decision was reached based on consultation.
Typical immunohistochemical staining pattern of p53 (a–c) and PD-L1 (d, e). a Normal staining pattern of nuclei in tumour adjacent pancreatic tissue and “negative” staining pattern in PDAC for p53 in IHC. b Abnormal staining pattern in PDAC; nuclear accumulation of p53 protein was observed in IHC, which was defined as “Positive” indicating mutated p53. C, Absence of p53 in PDAC, which was also defined as “Positive”. Example of typical immunohistochemical “Positive” and “Negative” staining pattern of PD-L1, respectively (d, e). Image magnification of ×400.
CT acquisition
All CTs were performed using a 128-detector-row CT system (SOMATOM Definition Flash; Siemens; Erlangen, Germany). The following imaging parameters were applied: tube voltage, 120 kVp; tube current, 160 mAs; beam pitch, 0.6; and resolution 0.68 × 0.68 × 5 mm. Contrast agent (Iopamidol, Iopamiron 300; Bayer, Leverkusen, Germany; 100 mL) was administered through the superficial vein of upper extremity using double-head power injector (body weight ≥ 55 kg; 150 ml injected at 4.5 mL/s, body weight < 55 kg; 100 ml injected at 3.6 ml/s). The application of contrast agent was followed by that of normal saline (30 mL) at the same injection rate. After injection of the contrast agent, two-phase images of imaging slices taken at 40 and 120 s were used for analysis.
Tumour segmentation
A board-certified diagnostic radiologist and surgeon (15 and 7 years of experience in pancreatic imaging, respectively) delineated the volume of interest in pancreatic cancer (VOIpc) in early- and late-phase images individually (Fig. 2).
If available, magnetic resonance imaging and 18F-positron emission tomography were referenced. Next, VOI+4mm was created by mechanically expanding the axial plane only by 4 mm around each VOIpc. VOI+4mm included the tumour and peritumoural region seen in CT.
Imaging feature extraction
Imaging features were extracted using an open-source python package, PyRadiomics v2.2.0 (http://www.radiomics.io/pyradiomics.html).18 Pyradiomics can calculate various quantitative values from images using various mathematical methods based on morphological, histogram and texture analyses. The quantitative values reflect the imaging characteristics of the tumour, such as heterogeneity. Absolute rescaling method (−150 to 500 Hounsfield unit) was applied. Pixel values between the upper and lower limits were resampled into 64 levels and those outside the limits were truncated. Morphology, histogram, and texture features were calculated from original images. The same types of features were extracted from the Laplacian of Gaussian-filtered and wavelet-transformed images. Finally, a total of 1037 features were extracted from each VOI (Fig. 2).
Variable definitions
For statistical survival analysis, age, preoperative carcinoembryonic antigen (CEA), and preoperative CA19-9 were divided into two groups with 70, 3.3, and 137.4 as the median, respectively. For surgery time and blood loss, we compared the survival rates in both groups (311 min, 600 ml). We performed pancreaticoduodenectomy (PD), distal pancreatectomy (DP), and total pancreatectomy (TP) for PDAC. In addition, we adopted a unified procedure at the Department of Hepatobiliary and Pancreatic Surgery in our hospital. Lymph nodes, margin status, cytology, lymphatic invasion, neural invasion, vascular invasion, differentiation, and TNM staging (UICC 8th edition) were defined based on the pathological results. Lymph nodes were either positive or negative for lymph node metastasis. Vascular invasion was divided into two groups: v0, v1 and v2, v3, since there was only one patient in v0. Differentiation was divided into two groups for survival analysis, namely “well” and “moderate/poor”.
Statistics
Significance of the difference between the status of p53, PD-L1 (positive/negative), and several clinical and pathologic variables was assessed by the χ2 test, Fisher’s exact test, or Mann–Whitney U test. Overall survival (OS) was defined as the period between surgery and final observation (in days). A survival curve was prepared using the Kaplan–Meier method, and log-rank test assessed the significant differences. Multivariate analysis was performed using the Cox regression model to study the significant factors in log-rank test. A P-value < 0.050 was considered significant.
Machine learning
Feature selection consisted of two steps to stabilise the predictive power of the model. First, Mann–Whitney U test was performed on each imaging feature, and only those with significant difference were retained. Second, another feature selection with recursive feature elimination was performed using random forest function. Finally, 2074 features derived from early and late phases were put into XGBoost to construct the predictive models for p53 and PD-L1, respectively. The feature selection and model construction steps were performed with nested cross validation. Inner cross validation for feature selection was 5-repeat 5-fold and outer cross validation for model construction was 10-repeat 5-fold (Fig. 2).
Model evaluation
The mean output values of 10 repeats were used for receiver operating characteristic (ROC) analysis. To evaluate the survival prediction of machine learning models, cut-off values were defined from the point closest to the top-left part of ROC plot with perfect sensitivity and specificity. Log-rank test was performed between two groups, defined by predicted p53 and PD-L1, respectively. All statistical analyses and machine-learning were conducted using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). The summary of the processing is shown in Fig. 2.
Results
Patient background
From January 2013 to March 2018, 140 patients were diagnosed with pancreatic cancer, after surgery, based on pathological diagnosis. Of those, 22 patients were excluded, since they had received preoperative chemotherapy or chemoradiation. Four cases were excluded owing to atypical pancreatic cancer. Three cases were diagnosed as having intra-ductal papillary mucinous carcinoma with infiltration components; since the infiltration site was slight, the remaining samples could not be evaluated. One patient was excluded owing to liver metastasis at the time of surgery. The following cases were also excluded: one patient with oesophageal cancer, one with gastric cancer, and one with pancreatic recurrence referred to the current hospital from another. A retrospective study was conducted on 107 out of 140 subjects. The observation period was from January 2013 to July 2019, with a median of 708 days (58–2067 days). The median age was 70 years (50–87 years), and gender ratio was 60:47. There were 70 cases with PD (Pancreaticoduodenectomy), 35 cases with DP (Distal Pancreatectomy), and two cases with TP (Total Pancreatectomy). The median operation time was 311 min (121–586 min) and median blood loss was 600 ml (35–4600 ml). The median values for preoperative CEA and CA19-9 were 3.3 (0.5–47.3) and 138.4 (0–47588.2), respectively. Ninety-three patients were negative for intraoperative peritoneal washing cytology. The curative resection was R0 in 89 cases, and the histological types were well, moderate, and poor in 46, 53, and 8 cases, respectively. Lymphatic infiltration (ly0) and nerve infiltration (ne0) were negative in 29 and 6 cases, respectively. Venous invasion was negative in only 1 case, and v1 were 20 cases. Lymph node metastasis was found in 76 cases. In T factor (UICC 8th edition), T2 was the maximum in 60 cases. In TNM classification (UICC 8th edition), Stage III was the most common in 39 cases, followed by Stage III in 37 cases (Table 1).
p53 immunostaining
Seventy-five cases (70.0%) were p53 positive. There was a difference in gender ratio between p53 positive and negative (P = 0.036); however, there was no difference in other clinicopathological factors such as preoperative tumour markers and lymph node metastasis (Table 1).
PD-L1 immunostaining
Thirty-six cases (33.6%) were PD-L1 positive. Lymph node metastasis was more frequent in the PD-L1-positive group (P < 0.001), and a tendency for higher stage cases in PD-L1-positive group was observed in TNM staging (UICC 8th edition). There was no difference between other factors, including histological types, in PD-L1 (Table 1).
Relationship between clinicopathological factors and prognosis
We examined the relationship between clinicopathological factors and prognosis using the log-rank test. There was no difference in survival rates based on gender or age (Table 2). CEA and CA19-9, known as preoperative tumour markers, were analysed and the high-CA19-9 group was significantly related to poor prognosis in PDAC (P = 0.004). In the surgical procedure, DP/TP and long-term operation significantly worsened prognosis (P = 0.001, 0.001). Intraoperative peritoneal washing cytology and margin status histology showed no clear deterioration in prognosis, although the latter significantly worsened in venous invasion and nerve invasion groups (P = 0.013, 0.043). Moreover, prognosis was significantly worse in the lymph node metastasis-positive group and T3 group (UICC 8th edition) (P < 0.010, 0.018), and similar tendency was observed in TNM staging (Table 2).
Relationship between p53 and PD-L1 expression (by immunostaining) and prognosis
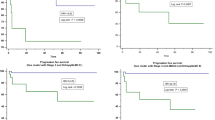
We investigated the correlation between clinicopathological factors, including p53 and PD-L1 expression, and prognosis. The p53-positive and PD-L1-positive groups were associated with poor prognosis (P = 0.008, 0.013) (Fig. 3a, b).
Kaplan–Meier plots for patients with PDAC demonstrating prognostic influence for the “real” status (positive/negative) of p53 in IHC (a), PD-L1 in IHC (b) and “predicted” status of p53 (c), PD-L1 (d) with reference to overall survival. ROC curve was constructed by “real” status and “predicted” status of p53 (e) and PD-L1 (f), respectively. “predicted” status was calculated with machine learning and 1037 IFs extracted from CT.
Examination of prognostic factors by multivariate analysis
In the 107 patients studied, we found p53, PD-L1, CA19-9, surgical time, operative procedure, venous invasion-positive, nerve invasion-positive, lymph node metastasis, and T factors to be significantly associated with poor prognosis; of these nine factors, six with the lowest P values (p53, PD-L1, CA19-9, operation time, operative procedure, and lymph node metastasis) underwent multivariate analysis. All factors except the operation time were found to be significantly associated with poor prognosis (Table 3).
Predictive power of machine learning models
Area under the curve (AUC) for p53 was 0.705 and 0.795 with imaging features of VOIpc and VOI+4mm, respectively (Fig. 3e). The AUC for PD-L1 was 0.660 and 0.683 with imaging features of VOIpc and VOI+4mm, respectively (Fig. 3f). Overall survival was significantly different between the groups defined by the predicted status of p53 (positive/negative) using machine learning (P = 0.015) (Fig. 2c). In contrast, the predicted status of PD-L1 divided the groups into better and worse prognosis, although the difference was not significant (P = 0.096) (Fig. 3d).
Discussion
We confirmed the positive expression of abnormal p53 and PD-L1 in IHC to be an independent prognostic factor in PDAC. Furthermore, we examined whether the expression of p53 and PD-L1 could be predicted using radiogenomic analysis. Results clearly revealed the expression of p53 to be predictable with certain accuracy.
Although p53 regulates the cell cycle, DNA-damaged cells can lead to apoptosis. When p53 is mutated, it abnormally proliferates and participates in carcinogenesis. Approximately 70–90% of p53 mutations are found in PDAC, and it is believed that if a therapeutic drug for p53 was available, it would have a great effect on PDAC.2,19 Recently, there have been reports of agents that restore p53 function, showing effectiveness in oesophageal squamous cell carcinoma, osteosarcoma, multiple myeloma, lung cancer, breast cancer, and colon cancer in vitro.20,21,22,23,24
In this study, we used immunohistochemistry to determine the presence of p53 mutations. In patients with PDAC, the rate of abnormal expression of p53, as seen by immunohistochemistry, was reported to be 50–90%, whereas Ohshima et al. had reported it to be 81.8%.2,3,4,25 In this study, positive p53 was seen in 75 cases (70%), almost in agreement with the previous report.4,26 Lack of p53 protein (null type) is associated with frameshift and nonsense mutations, whereas p53 over expression occurs (overexpression type) as a result of missense mutations.27,28 Further, in the case of pancreatic cancer, Schlitter et al. showed a relationship between p53 mutations and a p53 positive status on IHC (null type and overexpression type).26 In their study, p53 mutations were found overall in 77.6% of the PDAC cases. Of these, 26.6% were null type, which were associated with intragenic deletions, nonsense mutations, frameshifts, or splice site mutations in p53, while 51% were overexpression type, which were associated with missense mutations.26 The results indicated that a p53 positive status on IHC reflects the presence of p53 gene mutations.26 We found similar frequencies of p53-positive PDAC cases in our study—null and overexpression types in 20 (18.7%) and 55 (51.3%) cases, respectively. Other studies have also shown that p53 positive status on IHC correlates with p53 mutations. In fact, for colon, breast, ovarian, and bladder cancer, p53 mutations have been defined by p53 positive status on IHC, and this information has been used to predict prognosis in these cancers.4,28,29,30,31,32,33
There is no consensus yet for demonstrating and quantifying PD-L1 expression in PDAC, although the expression rate of PD-L1 in tumour cells is regarded as ~4–60%; however, there is no clear threshold to define PD-L1 positivity.10,34 In this study, we defined PD-L1-positive cases as the ones in which IHC-stained cells were 1% or more of all tumour cells; the group of PD-L1-positive cases is correlated with vascular invasion, histological type, and lymph node metastasis, although the reports have not been consistent. Results of the current study correlated with lymph node metastasis.1,6 PD-L1 is a marker for ICIs that have shown significant results in lung cancer.5,7,8 In recent years, ICI has been suggested to have an effect on PDAC, and hence, has attracted remarkable attention. However, clinical trials have shown the clinical effect of anti-PD-L1 antibody alone to be of little benefit, although the number of cases reported was small.35 Recent reports have indicated ICI to be effective in colorectal cancer with high frequency microsatellite instability (MSI-H), known to be present in ~1.5% of all colorectal cancers.36 The instability may be expected to occur in ~2% of PDACs as well, and the effect of ICI may also be expected similarly. It may be effective when administered to patients with high PD-L1 expression or when combined with other molecular targeting drugs, chemotherapy, or radiation therapy.
As described above, it is obvious that p53 and PD-L1 may be important factors in the diagnosis and treatment of PDAC at present, as well as in the future. However, examining the expression and mutation of these factors using molecular biology techniques would require more time and expenses, owing to which an alternative imaging approach would be preferable. If this can be made feasible, it will have immense clinical significance. Radiogenomics is being increasingly reported in malignant tumours other than PDAC. In breast cancer, Genevieve et al. had predicted the Oncotype DX Test Recurrence Score from IFs extracted from mammography and MRI, and showed it to be a convenient biomarker for recurrence.10 Hectors et al. had reported the analysis of digital data in MRI to possibly predict the expression of vascular endothelial growth factor A (VEGFA) and immune checkpoints, differentiation cluster 274 (CD274), and cytotoxic T lymphocyte-associated protein 4 (CTLA4) in hepatocellular carcinoma.37 Taguchi et al. had reported KRAS mutations in colorectal cancer predicted from CT images using AI technology.38 A previous report had identified blood microRNAs associated with prognosis in oesophageal squamous cell carcinoma and predicted microRNA level and prognosis from IFs based on CT.39 Although there was only one report on pancreatic cancer, Attiyeh et al. reported that in 35 cases, only 28 IFs were significant for SMAD4, out of 255 IFs extracted from CT. Using a two-dimensional scale, difference in the distribution of SMAD4 between normal and abnormal cases could be demonstrated, and the status of SMAD4 (normal / abnormal) could be identified by CT; the proportion of stroma could also be predicted from IFs.15 However, the number of cases was 35, and the number of IFs used for analysis was strictly limited to 28. Although radiogenomic analysis had been performed by various approaches, there has been no report yet on radiogenomic analysis predicting genetic information from IFs using machine learning in pancreatic cancer.
In this study, it was possible to predict p53 status (positive/negative) with some accuracy using IFs extracted from CT images and radiomics analysis. However, AUC was not sufficient with VOIpc set at the tumour margin of PDAC, and a sufficient AUC could be obtained with VOI+4mm set in the region including the tumour periphery area and additional 4 mm. This implied that VOIpc, which captured only the outline of tumour in the CT image, might reflect only the solid content of PDAC, and might not include all the tumour with the peripheral invasive part. Hutchings et al. had suggested p53 mutation to be associated with carcinogenesis, spread, and progression to the pancreatic duct surrounding PDAC.40 Moreover, other reports had suggested p53 mutation to promote tumour cell invasion and metastasis via cancer-associated fibroblasts.27 These results indicated p53 mutation to possibly affect the major margins. Therefore, representing such properties of p53 and including the surroundings around the outline of tumour image might have caused the enhanced detection sensitivity in radiomics analysis.
The PD-L1 status (positive/negative) could not be adequately predicted by image properties (AUC = 0.660); predictions including analysis around the tumour (+4 mm) were also not sufficient (AUC = 0.683). An earlier report had suggested PD-L1 to affect histology, lymph node metastasis, and vascular invasion; however, it was inconsistent and reported PD-L1 to affect the tumour microenvironment.1,6 On the other hand, there was no report regarding the effects on extratumoural progression.34,35,41,42 The features of PD-L1-positive/-negative cases may not be well reflected in CT images. While p53 is a molecule that exists upstream in many genetic cascades (also called “genomic guardian”) and has multiple functions in cancer suppression, PD-L1 is a downstream molecule with the distinct function of immunosuppression.43 This difference may introduce a discrepancy in their influence on the image.
This study had some limitations, first being the small number of cases (107 cases). Second, this was a retrospective study performed at a single centre. Larger prospective studies in collaboration with other centres should be conducted to further confirm the results of our study. In addition, p53 gene mutations were evaluated only indirectly using IHC. In the future, the frequency and type of p53 mutations present in PDACs could be directly assessed using next-generation sequencing.
Here, we used only CT images for PDAC using radiomics analysis, whereas PDAC may have many indistinguishable lesions and may require image information from another modality with high contrast resolution such as MRI. Although VOIs were created by skilled radiologists and surgeons, and PDAC had many unclearly defined lesions, VOIs might have been chosen with bias, which could be an issue in future considerations.
Conclusion
In this study, occurrence of p53 gene mutation and abnormal PD-L1 expression was examined using IHC. Positive p53 and PD-L1 were independent factors that worsened prognosis. Radiogenomic analysis using CT images was able to predict the presence of p53 mutations, and hence, disease prognosis. Prediction of genetic information from these images using radiogenomics may help both precision and personalised medicine.
References
Spath, C., Nitsche, U., Muller, T., Michalski, C., Erkan, M., Kong, B. et al. Strategies to improve the outcome in locally advanced pancreatic cancer. Minerva Chir. 70, 97–106 (2015).
Waddell, N., Pajic, M., Patch, A. M., Chang, D. K., Kassahn, K. S., Bailey, P. et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518, 495–501 (2015).
Kamisawa, T., Wood, L. D., Itoi, T. & Takaori, K. Pancreatic cancer. Lancet (Lond., Engl.) 388, 73–85 (2016).
Oshima, M., Okano, K., Muraki, S., Haba, R., Maeba, T., Suzuki, Y. et al. Immunohistochemically detected expression of 3 major genes (CDKN2A/p16, TP53, and SMAD4/DPC4) strongly predicts survival in patients with resectable pancreatic cancer. Ann. Surg. 258, 336–346 (2013).
Reck, M., Rodriguez-Abreu, D., Robinson, A. G., Hui, R., Csoszi, T., Fulop, A. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833 (2016).
Gao, H. L., Liu, L., Qi, Z. H., Xu, H. X., Wang, W. Q., Wu, C. T. et al. The clinicopathological and prognostic significance of PD-L1 expression in pancreatic cancer: a meta-analysis. Hepatobiliary Pancreat. Dis. Int. 17, 95–100 (2018).
Brahmer, J., Reckamp, K. L., Baas, P., Crino, L., Eberhardt, W. E., Poddubskaya, E. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373, 123–135 (2015).
Borghaei, H., Paz-Ares, L., Horn, L., Spigel, D. R., Steins, M., Ready, N. E. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639 (2015).
Herskind, C., Talbot, C. J., Kerns, S. L., Veldwijk, M. R., Rosenstein, B. S. & West, C. M. Radiogenomics: a systems biology approach to understanding genetic risk factors for radiotherapy toxicity? Cancer Lett. 382, 95–109 (2016).
Woodard, G. A., Ray, K. M., Joe, B. N. & Price, E. R. Qualitative radiogenomics: association between oncotype DX Test recurrence score and BI-RADS mammographic and breast MR imaging features. Radiology 286, 60–70 (2018).
Zhou, M., Leung, A., Echegaray, S., Gentles, A., Shrager, J. B., Jensen, K. C. et al. Non-small cell lung cancer radiogenomics map identifies relationships between molecular and imaging phenotypes with prognostic implications. Radiology 286, 307–315 (2018).
Kickingereder, P., Bonekamp, D., Nowosielski, M., Kratz, A., Sill, M., Burth, S. et al. Radiogenomics of glioblastoma: machine learning-based classification of molecular characteristics by using multiparametric and multiregional MR imaging features. Radiology 281, 907–918 (2016).
Eilaghi, A., Baig, S., Zhang, Y., Zhang, J., Karanicolas, P., Gallinger, S. et al. CT texture features are associated with overall survival in pancreatic ductal adenocarcinoma—a quantitative analysis. BMC Med. Imaging 17, 38 (2017).
Sun, R., Limkin, E. J., Vakalopoulou, M., Dercle, L., Champiat, S., Han, S. R. et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 19, 1180–1191 (2018).
Attiyeh, M. A., Chakraborty, J., McIntyre, C. A., Kappagantula, R., Chou, Y., Askan, G. et al. CT radiomics associations with genotype and stromal content in pancreatic ductal adenocarcinoma. Abdom. Radiol. 44, 3148–3157 (2019).
Attiyeh, M. A., Chakraborty, J., Doussot, A., Langdon-Embry, L., Mainarich, S., Gonen, M. et al. Survival prediction in pancreatic ductal adenocarcinoma by quantitative computed tomography image analysis. Ann. surgical Oncol. 25, 1034–1042 (2018).
Kobel, M., Ronnett, B. M., Singh, N., Soslow, R. A., Gilks, C. B. & McCluggage, W. G. Interpretation of P53 immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int. J. Gynecol. Pathol. 38, S123–S131 (2019).
van Griethuysen, J. J. M., Fedorov, A., Parmar, C., Hosny, A., Aucoin, N., Narayan, V. et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 77, e104–e107 (2017).
Feldser, D. M., Kostova, K. K., Winslow, M. M., Taylor, S. E., Cashman, C., Whittaker, C. A. et al. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature 468, 572–575 (2010).
Furukawa, H., Makino, T., Yamasaki, M., Tanaka, K., Miyazaki, Y., Takahashi, T. et al. PRIMA-1 induces p53-mediated apoptosis by upregulating Noxa in esophageal squamous cell carcinoma with TP53 missense mutation. Cancer Sci. 109, 412–421 (2018).
Saha, M. N., Jiang, H., Yang, Y., Reece, D. & Chang, H. PRIMA-1Met/APR-246 displays high antitumor activity in multiple myeloma by induction of p73 and Noxa. Mol. Cancer Ther. 12, 2331–2341 (2013).
Zandi, R., Selivanova, G., Christensen, C. L., Gerds, T. A., Willumsen, B. M. & Poulsen, H. S. PRIMA-1Met/APR-246 induces apoptosis and tumor growth delay in small cell lung cancer expressing mutant p53. Clin. Cancer Res. 17, 2830–2841 (2011).
Liang, Y., Besch-Williford, C. & Hyder, S. M. PRIMA-1 inhibits growth of breast cancer cells by re-activating mutant p53 protein. Int. J. Oncol. 35, 1015–1023 (2009).
Li, X. L., Zhou, J., Chan, Z. L., Chooi, J. Y., Chen, Z. R. & Chng, W. J. PRIMA-1met (APR-246) inhibits growth of colorectal cancer cells with different p53 status through distinct mechanisms. Oncotarget 6, 36689–36699 (2015).
Thiem, A., Hesbacher, S., Kneitz, H., di Primio, T., Heppt, M. V., Hermanns, H. M. et al. IFN-gamma-induced PD-L1 expression in melanoma depends on p53 expression. J. Exp. Clin. Cancer Res. 38, 397 (2019).
Schlitter, A. M., Segler, A., Steiger, K., Michalski, C. W., Jager, C., Konukiewitz, B. et al. Molecular, morphological and survival analysis of 177 resected pancreatic ductal adenocarcinomas (PDACs): identification of prognostic subtypes. Sci. Rep. 7, 41064 (2017).
Vennin, C., Melenec, P., Rouet, R., Nobis, M., Cazet, A. S., Murphy, K. J. et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat. Commun. 10, 3637 (2019).
Muller, P. A. & Vousden, K. H. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell 25, 304–317 (2014).
Murnyak, B. & Hortobagyi, T. Immunohistochemical correlates of TP53 somatic mutations in cancer. Oncotarget 7, 64910–64920 (2016).
Kaserer, K., Schmaus, J., Bethge, U., Migschitz, B., Fasching, S., Walch, A. et al. Staining patterns of p53 immunohistochemistry and their biological significance in colorectal cancer. J. Pathol. 190, 450–456 (2000).
Yemelyanova, A., Vang, R., Kshirsagar, M., Lu, D., Marks, M. A., Shih, Ie. M. et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod. Pathol. 24, 1248–1253 (2011).
Hodgson, A., Xu, B. & Downes, M. R. p53 immunohistochemistry in high-grade urothelial carcinoma of the bladder is prognostically significant. Histopathology 71, 296–304 (2017).
Alsner, J., Jensen, V., Kyndi, M., Offersen, B. V., Vu, P., Borresen-Dale, A. L. et al. A comparison between p53 accumulation determined by immunohistochemistry and TP53 mutations as prognostic variables in tumours from breast cancer patients. Acta Oncol. 47, 600–607 (2008).
Herbst, R. S., Soria, J. C., Kowanetz, M., Fine, G. D., Hamid, O., Gordon, M. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Teng, M. W., Ngiow, S. F., Ribas, A. & Smyth, M. J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 75, 2139–2145 (2015).
Le, D. T., Durham, J. N., Smith, K. N., Wang, H., Bartlett, B. R., Aulakh, L. K. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017).
Hectors, S. J., Wagner, M., Bane, O., Besa, C., Lewis, S., Remark, R. et al. Quantification of hepatocellular carcinoma heterogeneity with multiparametric magnetic resonance imaging. Sci. Rep. 7, 2452 (2017).
Taguchi, N., Oda, S., Yokota, Y., Yamamura, S., Imuta, M., Tsuchigame, T. et al. CT texture analysis for the prediction of KRAS mutation status in colorectal cancer via a machine learning approach. Eur. J. Radiol. 118, 38–43 (2019).
Hoshino, I., Yokota, H., Ishige, F., Iwatate, Y., Takeshita, N., Nagase, H. et al. Radiogenomics predicts the expression of microRNA-1246 in the serum of esophageal cancer patients. Sci. Rep. 10, 2532 (2020).
Hutchings, D., Waters, K. M., Weiss, M. J., Wolfgang, C. L., Makary, M. A., He, J. et al. Cancerization of the pancreatic ducts: demonstration of a common and under-recognized process using immunolabeling of paired duct lesions and invasive pancreatic ductal adenocarcinoma for p53 and Smad4 expression. Am. J. surgical Pathol. 42, 1556–1561 (2018).
Snyder, A., Makarov, V., Merghoub, T., Yuan, J., Zaretsky, J. M., Desrichard, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Lane, D. P. Cancer. p53, guardian of the genome. Nature 358, 15–16 (1992).
Author information
Authors and Affiliations
Contributions
Y.I. and I.H. analysed and interpreted patient data. Y.I. was a major contributor to the writing of the paper. F.I., S.C., H.A., H.Yanagibashi., H.N., and W.T. performed the CT scans in PDAC patients and registered the images. H.Yokota and Y.M. extracted the imaging features from CT scans and analysed the relationship between imaging features and clinicopathological features. M.I. performed the pathological examination of pancreatic cancer samples and interpreted IHC results. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Chiba Cancer Center Review Board (H29-006). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all patients in this study.
Consent to publish
Written informed consent was obtained from the patients for publication of this study and accompanying clinicopathological data.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Funding information
Funding information is not applicable.
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iwatate, Y., Hoshino, I., Yokota, H. et al. Radiogenomics for predicting p53 status, PD-L1 expression, and prognosis with machine learning in pancreatic cancer. Br J Cancer 123, 1253–1261 (2020). https://doi.org/10.1038/s41416-020-0997-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-0997-1
This article is cited by
-
Radiogenomic analysis of prediction HER2 status in breast cancer by linking ultrasound radiomic feature module with biological functions
Journal of Translational Medicine (2023)
-
Prediction of p53 mutation status in rectal cancer patients based on magnetic resonance imaging-based nomogram: a study of machine learning
Cancer Imaging (2023)
-
Immunohistochemical analysis of a panel of cancer stem cell markers and potential therapeutic markers in pancreatic ductal adenocarcinoma
Journal of Cancer Research and Clinical Oncology (2023)
-
Radiomics Based on Contrast-Enhanced CT for Recognizing c-Met-Positive Hepatocellular Carcinoma: a Noninvasive Approach to Predict the Outcome of Sorafenib Resistance
Molecular Imaging and Biology (2023)
-
Integrated analysis of tertiary lymphoid structures in relation to tumor-infiltrating lymphocytes and patient survival in pancreatic ductal adenocarcinoma
Journal of Gastroenterology (2023)