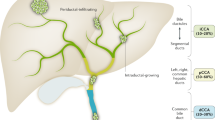
Abstract
Biliary tract cancers (BTCs) are a group of rare and aggressive malignancies that arise in the biliary tree within and outside the liver. Beyond surgical resection, which is beneficial for only a small proportion of patients, current strategies for treating patients with BTCs include chemotherapy, as a single agent or combination regimens, in the adjuvant and palliative setting. Increased characterisation of the molecular landscape of these tumours has facilitated the identification of molecular vulnerabilities, such as IDH mutations and FGFR fusions, that can be exploited for the treatment of BTC patients. Beyond targeted therapies, active research avenues explore the development of novel therapeutics that target the crosstalk between cancer and stroma, the cellular pathways involved in the regulation of cell death, the chemoresistance phenotype and the dysregulation of RNA. In this review, we discuss the therapeutic opportunities currently available in the management of BTC patients, and explore the strategies that can support the implementation of precision oncology in BTCs, including novel molecular targets, liquid biopsies and patient-derived predictive tools.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Change history
05 July 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41416-020-01243-3
References
Khan, S. A., Tavolari, S. & Brandi, G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 39, 19–31 (2019).
Saha, S. K., Zhu, A. X., Fuchs, C. S. & Brooks, G. A. Forty‐year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist 21, 594–599 (2016).
Kim, V. N., Han, J. & Siomi, M. C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 10, 126–139 (2009).
Bridgewater, J., Lopes, A., Palmer, D., Cunningham, D., Anthoney, A., Maraveyas, A. et al. Quality of life, long-term survivors and long-term outcome from the ABC-02 study. Br. J. Cancer 114, 965–971 (2016).
Horgan, A. M., Amir, E., Walter, T. & Knox, J. J. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J. Clin. Oncol. 30, 1934–1940 (2012).
Ebata, T., Hirano, S., Konishi, M., Uesaka, K., Tsuchiya, Y., Ohtsuka, M. et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br. J. Surg. 105, 192–202 (2018).
Edeline, J., Benabdelghani, M., Bertaut, A., Watelet, J., Hammel, P., Joly, J.-P. et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase III study. J. Clin. Oncol. 37, 658–667 (2019).
Primrose, J. N., Fox, R. P., Palmer, D. H., Malik, H. Z., Prasad, R., Mirza, D. et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 20, 663–673 (2019).
Lamarca, A., Edeline, J., McNamara, M. G., Hubner, R. A., Nagino, M., Bridgewater, J. et al. Current standards and future perspectives in adjuvant treatment for biliary tract cancers. Cancer Treat. Rev. 84, 101936 (2020).
Valle, J., Wasan, H., Palmer, D. H., Cunningham, D., Anthoney, A., Maraveyas, A. et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 362, 1273–1281 (2010).
Okusaka, T., Nakachi, K., Fukutomi, A., Mizuno, N., Ohkawa, S., Funakoshi, A. et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br. J. Cancer 103, 469–474 (2010).
Morizane, C., Okusaka, T., Mizusawa, J., Katayama, H., Ueno, M., Ikeda, M. et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann. Oncol. 30, 1950–1958 (2019).
Perkhofer, L., Berger, A. W., Beutel, A. K., Gallmeier, E., Angermeier, S., Fischer von Weikersthal, L. et al. Nal-IRI with 5-fluorouracil (5-FU) and leucovorin or gemcitabine plus cisplatin in advanced biliary tract cancer - the NIFE trial (AIO-YMO HEP-0315) an open label, non-comparative, randomized, multicenter phase II study. BMC Cancer 19, 990 (2019).
Shroff, R. T., Javle, M. M., Xiao, L., Kaseb, A. O., Varadhachary, G. R., Wolff, R. A. et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers. JAMA Oncol. 5, 824–830 (2019).
Lamarca, A., Ross, P., Wasan, H. S., Hubner, R. A., McNamara, M. G., Lopes, A. et al. Advanced intrahepatic cholangiocarcinoma: post hoc analysis of the ABC-01, -02, and -03 clinical trials. JNCI J. Natl. Cancer Inst. 112, 200–210 (2019).
Edeline, J., Touchefeu, Y., Guiu, B., Farge, O., Tougeron, D., Baumgaertner, I. et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma. JAMA Oncol. 6, 51 (2020).
Cercek, A., Boerner, T., Tan, B. R., Chou, J. F., Gönen, M., Boucher, T. M. et al. Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma. JAMA Oncol. 6, 60 (2020).
Leone, F., Filippi, R., Palloni, A., Fornaro, L., Casadei Gardini, A., Aprile, G. et al. Prognostic factors in unresectable biliary tract cancer: a GICO (Gruppo Italiano COlangiocarcinoma) retrospective analysis. Ann. Oncol. 28, vi48 (2017).
Lamarca, A., Hubner, R. A., David Ryder, W. & Valle, J. W. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann. Oncol. 25, 2328–2338 (2014).
Valle, J. W., Borbath, I., Khan, S. A., Huguet, F., Gruenberger, T. & Arnold, D. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27, v28–v37 (2016).
Bridgewater, J., Palmer, D., Cunningham, D., Iveson, T., Gillmore, R., Waters, J. et al. Outcome of second-line chemotherapy for biliary tract cancer. Eur. J. Cancer 49, 1511 (2013).
Brieau, B., Dahan, L., De Rycke, Y., Boussaha, T., Vasseur, P., Tougeron, D. et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: a large multicenter study by the Association des Gastro-Entérologues Oncologues. Cancer 121, 3290–3297 (2015).
Schweitzer, N., Kirstein, M. M., Kratzel, A., Mederacke, Y., Fischer, M., Manns, M. P. et al. Second‐line chemotherapy in biliary tract cancer: Outcome and prognostic factors. Liver Int. 39, 914–923 (2019).
Lamarca, A., Palmer, D. H., Wasan, H. S., Ross, P. J., Ma, Y. T., Arora, A. et al. ABC-06 | A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced / metastatic biliary tract cancers (ABC) previously-tr. J. Clin. Oncol. 37, 4003–4003 (2019).
Belkouz, A., Vos-Geelen, J., de, Eskens, F., Mathot, R. A. A., van Gulik, T., van Oijen, M. G. H. et al. Efficacy and safety of FOLFIRINOX in advanced biliary tract cancer after failure of gemcitabine plus cisplatin: a phase II trial. J. Clin. Oncol. 37, 4086–4086 (2019).
Pape, U.-F., Kasper, S., Meiler, J., Sinn, M., Vogel, A., Mueller, L. et al. Post-hoc analyses of a subgroup of patients with advanced biliary tract cancer (BTC) who crossed over to treatment with etoposide toniribate (EDO-S7.1) in a randomized phase II study. Ann. Oncol. 30, v278 (2019).
Wu, Y.-M., Su, F., Kalyana-Sundaram, S., Khazanov, N., Ateeq, B., Cao, X. et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 3, 636–647 (2013).
Abou-Alfa, G. K., Macarulla Mercade, T., Javle, M., Kelley, R. K., Lubner, S., Adeva, J. et al. ClarIDHy: a global, phase III, randomized, double-blind study of ivosidenib (IVO) vs placebo in patients with advanced cholangiocarcinoma (CC) with an isocitrate dehydrogenase 1 (IDH1) mutation. Ann. Oncol. 30, v872–v873 (2019).
Javle, M., Lowery, M., Shroff, R. T., Weiss, K. H., Springfeld, C., Borad, M. J. et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J. Clin. Oncol. 36, 276–282 (2018).
Mazzaferro, V., El-Rayes, B. F., Droz dit Busset, M., Cotsoglou, C., Harris, W. P., Damjanov, N. et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 120, 165–171 (2019).
Vogel, A., Sahai, V., Hollebecque, A., Vaccaro, G., Melisi, D., Al-Rajabi, R. et al. FIGHT-202: a phase II study of pemigatinib in patients (pts) with previously treated locally advanced or metastatic cholangiocarcinoma (CCA). Ann. Oncol. 30, v876 (2019).
Goyal, L., Bahleda, R., Furuse, J., Valle, J. W., Moehler, M. H., Oh, D.-Y. et al. FOENIX-101: A phase II trial of TAS-120 in patients with intrahepatic cholangiocarcinoma harboring FGFR2 gene rearrangements. J. Clin. Oncol. 37, TPS468–TPS468 (2019).
Chen, Y.-Y., Park, J. O., Su, W.-C., Oh, D.-Y., Kim, K.-P., Feng, Y.-H. et al. Preliminary results of a ph2a study to evaluate the clinical efficacy and safety of erdafitinib in Asian patients with biomarker-selected advanced cholangiocarcinoma (CCA). Ann. Oncol. 29, viii209 (2018).
Hyman, D. M., Goyal, L., Grivas, P., Meric-Bernstam, F., Tabernero, J., Hu, Y. et al. FUZE clinical trial: a phase 2 study of Debio 1347 in FGFR fusion-positive advanced solid tumors irrespectively of tumor histology. J. Clin. Oncol. 37, TPS3157–TPS3157 (2019).
Meric-Bernstam, F., Arkenau, H., Tran, B., Bahleda, R., Kelley, R., Hierro, C. et al. Efficacy of TAS-120, an irreversible fibroblast growth factor receptor (FGFR) inhibitor, in cholangiocarcinoma patients with FGFR pathway alterations who were previously treated with chemotherapy and other FGFR inhibitors. Ann. Oncol. 29, v100 (2018).
Javle, M. M., Borbath, I., Clarke, S. J., Hitre, E., Louvet, C., Mercade, T. M. et al. Infigratinib versus gemcitabine plus cisplatin multicenter, open-label, randomized, phase 3 study in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: The PROOF trial. J. Clin. Oncol. 37, TPS4155–TPS4155 (2019).
Nakamura, H., Arai, Y., Totoki, Y., Shirota, T., Elzawahry, A., Kato, M. et al. Genomic spectra of biliary tract cancer. Nat. Genet. 47, 1003–1010 (2015).
Goyal, L., Shi, L., Liu, L. Y., Fece de la Cruz, F., Lennerz, J. K., Raghavan, S. et al. TAS-120 overcomes resistance to ATP-competitive FGFR inhibitors in patients with FGFR2 fusion–positive intrahepatic cholangiocarcinoma. Cancer Discov. 9, 1064–1079 (2019).
Lamberti, D., Cristinziano, G., Porru, M., Leonetti, C., Egan, J. B., Shi, C. et al. HSP90 inhibition drives degradation of FGFR2 fusion proteins: implications for treatment of cholangiocarcinoma. Hepatology 69, hep.30127 (2018).
Lampis, A., Carotenuto, P., Vlachogiannis, G., Cascione, L., Hedayat, S., Burke, R. et al. MIR21 drives resistance to heat shock protein 90 inhibition in cholangiocarcinoma. Gastroenterology 154, 1066–1079.e5 (2018).
Cocco, E., Schram, A. M., Kulick, A., Misale, S., Won, H. H., Yaeger, R. et al. Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat. Med. 25, 1422–1427 (2019).
Gu, Y., Sai, Y., Wang, J., Yu, M., Wang, G., Zhang, L. et al. Preclinical pharmacokinetics, disposition, and translational pharmacokinetic/pharmacodynamic modeling of savolitinib, a novel selective cMet inhibitor. Eur. J. Pharm. Sci. 136, 104938 (2019).
Zhang, Z., Oyesanya, R. A., Campbell, D. J. W., Almenara, J. A., DeWitt, J. L. & Sirica, A. E. Preclinical assessment of simultaneous targeting of epidermal growth factor receptor (ErbB1) and ErbB2 as a strategy for cholangiocarcinoma therapy. Hepatology 52, 975–986 (2010).
Sia, D., Hoshida, Y., Villanueva, A., Roayaie, S., Ferrer, J., Tabak, B. et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology 144, 829–840 (2013).
Gao, Q., Zhao, Y., Wang, X., Guo, W., Gao, S., Wei, L. et al. Activating mutations in PTPN3 promote cholangiocarcinoma cell proliferation and migration and Are associated with tumor recurrence in patients. Gastroenterology 146, 1397–1407 (2014).
Yarlagadda, B., Kamatham, V., Ritter, A., Shahjehan, F. & Kasi, P. M. Trastuzumab and pertuzumab in circulating tumor DNA ERBB2-amplified HER2-positive refractory cholangiocarcinoma. npj Precis. Oncol. 3, 19 (2019).
Wainberg, Z. A., Lassen, U. N., Elez, E., Italiano, A., Curigliano, G., De Braud, F. G. et al. Efficacy and safety of dabrafenib (D) and trametinib (T) in patients (pts) with BRAF V600E–mutated biliary tract cancer (BTC): a cohort of the ROAR basket trial. J. Clin. Oncol. 37, 187–187 (2019).
Golan, T., Sella, T., O’Reilly, E. M., Katz, M. H. G., Epelbaum, R., Kelsen, D. P. et al. Overall survival and clinical characteristics of BRCA mutation carriers with stage I/II pancreatic cancer. Br. J. Cancer 116, 697–702 (2017).
Javle, M. M., Hainsworth, J. D., Swanton, C., Burris, H. A., Kurzrock, R., Sweeney, C. et al. Pertuzumab + trastuzumab for HER2-positive metastatic biliary cancer: Preliminary data from MyPathway. J. Clin. Oncol. 35, 402–402 (2017).
Braconi, C., Roessler, S., Kruk, B., Lammert, F., Krawczyk, M. & Andersen, J. B. Molecular perturbations in cholangiocarcinoma: Is it time for precision medicine? Liver Int. 39, 32–42 (2019).
Ikeda, M., Ohno, I., Ueno, H., Mitsunaga, S., Hashimoto, Y., Okusaka, T. et al. Phase I study of resminostat, an HDAC inhibitor, combined with S-1 in patients with pre-treated biliary tract or pancreatic cancer. Invest. New Drugs 37, 109–117 (2019).
Bang, Y.-J., Ueno, M., Malka, D., Chung, H. C., Nagrial, A., Kelley, R. K. et al. Pembrolizumab (pembro) for advanced biliary adenocarcinoma: results from the KEYNOTE-028 (KN028) and KEYNOTE-158 (KN158) basket studies. J. Clin. Oncol. 37, 4079–4079 (2019).
Marabelle, A., Le, D. T., Ascierto, P. A., Di Giacomo, A. M., De Jesus-Acosta, A., Delord, J.-P. et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 38, 1–10 (2020).
Ueno, M., Ikeda, M., Morizane, C., Kobayashi, S., Ohno, I., Kondo, S. et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet. Gastroenterol. Hepatol. 4, 611–621 (2019).
Arkenau, H., Martin‐Liberal, J., Calvo, E., Penel, N., Krebs, M. G., Herbst, R. S. et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced or metastatic biliary tract cancer: nonrandomized, open‐label, phase I trial (JVDF). Oncologist 23, 1407 (2018).
Marin, J. J. G., Lozano, E., Briz, O., Al-Abdulla, R., Serrano, M. A. & Macias, R. I. R. Molecular bases of chemoresistance in cholangiocarcinoma. Curr. Drug Targets 18, 889–900 (2017).
Marin, J. J. G., Lozano, E., Herraez, E., Asensio, M., Di Giacomo, S., Romero, M. R. et al. Chemoresistance and chemosensitization in cholangiocarcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 1444–1453 (2018).
Brandi, G., Deserti, M., Vasuri, F., Farioli, A., Degiovanni, A., Palloni, A. et al. Membrane localization of human equilibrative nucleoside transporter 1 in tumor cells may predict response to adjuvant gemcitabine in resected cholangiocarcinoma patients. Oncologist 21, 600–607 (2016).
Kim, J., Kim, H., Lee, J., Kim, J. W., Paik, W. H., Lee, S. H. et al. Human equilibrative nucleoside transporter 1 (hENT1) expression as a predictive biomarker for gemcitabine chemotherapy in biliary tract cancer. PLoS ONE 13, e0209104 (2018).
Martinez-Becerra, P., Vaquero, J., Romero, M. R., Lozano, E., Anadon, C., Macias, R. I. R. et al. No correlation between the expression of FXR and genes involved in multidrug resistance phenotype of primary liver tumors. Mol. Pharm. 9, 1693–1704 (2012).
Lozano, E., Macias, R. I. R., Monte, M. J., Asensio, M., Carmen, S., Sanchez‐Vicente, L. et al. Causes of hOCT1‐dependent cholangiocarcinoma resistance to sorafenib and sensitization by tumor‐selective gene therapy. Hepatology 70, 1246–1261 (2019).
Srimunta, U., Sawanyawisuth, K., Kraiklang, R., Pairojkul, C., Puapairoj, A. Titipungul, T. et al. High expression of ABCC1 indicates poor prognosis in intrahepatic cholangiocarcinoma. Asian Pac. J. Cancer Prev. 13, 125–130 (2012).
Chen, M.-H., Weng, J.-J., Cheng, C.-T., Wu, R.-C., Huang, S.-C., Wu, C.-E. et al. ALDH1A3, the major aldehyde dehydrogenase isoform in human cholangiocarcinoma cells, affects prognosis and gemcitabine resistance in cholangiocarcinoma patients. Clin. Cancer Res. 22, 4225–4235 (2016).
Nakajima, T., Takayama, T., Miyanishi, K., Nobuoka, A., Hayashi, T., Abe, T. et al. Reversal of multiple drug resistance in cholangiocarcinoma by the glutathione S-transferase-π-specific inhibitor O1-hexadecyl-γ-glutamyl- S-benzylcysteinyl-D-phenylglycine ethylester. J. Pharmacol. Exp. Ther. 306, 861–869 (2003).
Suksawat, M., Klanrit, P., Phetcharaburanin, J., Namwat, N., Khuntikeo, N., Titapun, A. et al. In vitro and molecular chemosensitivity in human cholangiocarcinoma tissues. PLoS ONE 14, e0222140 (2019).
Hahnvajanawong, C. Orotate phosphoribosyl transferase mRNA expression and the response of cholangiocarcinoma to 5-fluorouracil. World J. Gastroenterol. 18, 3955 (2012).
Habara, K., Ajiki, T., Kamigaki, T., Nakamura, T. & Kuroda, Y. High expression of thymidylate synthase leads to resistance to 5-fluorouracil in biliary tract carcinoma in vitro. Japanese J. Cancer Res. 92, 1127–1132 (2001).
Jimeno, A., Rubio-Viqueira, B., Amador, M. L., Oppenheimer, D., Bouraoud, N., Kulesza, P. et al. Epidermal growth factor receptor dynamics influences response to epidermal growth factor receptor targeted agents. Cancer Res. 65, 3003–3010 (2005).
Sato, J., Kimura, T., Saito, T., Anazawa, T., Kenjo, A., Sato, Y. et al. Gene expression analysis for predicting gemcitabine resistance in human cholangiocarcinoma. J. Hepatobiliary. Pancreat. Sci. 18, 700–711 (2011).
Ge, X., Wang, Y., Li, Q., Yu, H., Ji, G. & Miao, L. NK4 regulates 5-fluorouracil sensitivity in cholangiocarcinoma cells by modulating the intrinsic apoptosis pathway. Oncol. Rep. 30, 448–454 (2013).
Wattanawongdon, W., Hahnvajanawong, C., Namwat, N., Kanchanawat, S., Boonmars, T., Jearanaikoon, P. et al. Establishment and characterization of gemcitabine-resistant human cholangiocarcinoma cell lines with multidrug resistance and enhanced invasiveness. Int. J. Oncol. 47, 398–410 (2015).
Choodetwattana, P., Proungvitaya, S., Jearanaikoon, P. & Limpaiboon, T. The upregulation of OCT4 in acidic extracellular pH is associated with gemcitabine resistance in cholangiocarcinoma cell lines. Asian Pacific J. Cancer Prev. 20, 2745–2748 (2019).
Yamada, D., Kobayashi, S., Wada, H., Kawamoto, K., Marubashi, S., Eguchi, H. et al. Role of crosstalk between interleukin-6 and transforming growth factor-beta 1 in epithelial–mesenchymal transition and chemoresistance in biliary tract cancer. Eur. J. Cancer 49, 1725–1740 (2013).
Quintavalle, C., Burmeister, K., Piscuoglio, S., Quagliata, L., Karamitopoulou, E., Sepe, R. et al. High mobility group A1 enhances tumorigenicity of human cholangiocarcinoma and confers resistance to therapy. Mol. Carcinog. 56, 2146–2157 (2017).
Salati, M. & Braconi, C. Noncoding RNA in cholangiocarcinoma. Semin. Liver Dis. 39, 013–025 (2019).
Carotenuto, P., Hedayat, S., Fassan, M., Cardinale, V., Lampis, A., Guzzardo, V., et al. Modulation of biliary cancer chemo‐resistance through microRNA‐mediated rewiring of the expansion of CD133+ cells. Hepatology https://doi.org/10.1002/hep.31094 (2019).
Meng, F., Henson, R., Lang, M., Wehbe, H., Maheshwari, S., Mendell, J. T. et al. Involvement of Human Micro-RNA in Growth and Response to Chemotherapy in Human Cholangiocarcinoma Cell Lines. Gastroenterology 130, 2113–2129 (2006).
Peng, F., Jiang, J., Yu, Y., Tian, R., Guo, X., Li, X. et al. Direct targeting of SUZ12/ROCK2 by miR-200b/c inhibits cholangiocarcinoma tumourigenesis and metastasis. Br. J. Cancer 109, 3092–3104 (2013).
Okamoto, K., Miyoshi, K. & Murawaki, Y. miR-29b, miR-205 and miR-221 enhance chemosensitivity to gemcitabine in HuH28 human cholangiocarcinoma cells. PLoS ONE 8, e77623 (2013).
Chen, L., Yan, H.-X., Yang, W., Hu, L., Yu, L.-X., Liu, Q. et al. The role of microRNA expression pattern in human intrahepatic cholangiocarcinoma. J. Hepatol. 50, 358–369 (2009).
Jiao, D., Yan, Y., Shui, S., Wu, G., Ren, J., Wang, Y. et al. miR-106b regulates the 5-fluorouracil resistance by targeting Zbtb7a in cholangiocarcinoma. Oncotarget 8, 52913–52922 (2017).
Asukai, K., Kawamoto, K., Eguchi, H., Konno, M., Asai, A., Iwagami, Y. et al. Micro-RNA-130a-3p regulates gemcitabine resistance via PPARG in cholangiocarcinoma. Ann. Surg. Oncol. 24, 2344–2352 (2017).
Seehawer, M., Heinzmann, F., D’Artista, L., Harbig, J., Roux, P.-F., Hoenicke, L. et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature 562, 69–75 (2018).
Cadamuro, M., Brivio, S., Spirli, C., Joplin, R., Strazzabosco, M. & Fabris, L. Autocrine and paracrine mechanisms promoting chemoresistance in cholangiocarcinoma. Int. J. Mol. Sci. 18, 149 (2017).
Harnois, D. M., Que, F. G., Celli, A., LaRusso, N. F. & Gores, G. J. Bcl-2 is overexpressed and alters the threshold for apoptosis in a cholangiocarcinoma cell line. Hepatology 26, 884–890 (1997).
Minagawa, N., Kruglov, E. A., Dranoff, J. A., Robert, M. E., Gores, G. J. & Nathanson, M. H. The anti-apoptotic protein Mcl-1 inhibits mitochondrial Ca2+ Signals. J. Biol. Chem. 280, 33637–33644 (2005).
Yoon, H., Min, J.-K., Lee, J. W., Kim, D.-G. & Hong, H. J. Acquisition of chemoresistance in intrahepatic cholangiocarcinoma cells by activation of AKT and extracellular signal-regulated kinase (ERK)1/2. Biochem. Biophys. Res. Commun. 405, 333–337 (2011).
Wehrkamp, C. J., Gutwein, A. R., Natarajan, S. K., Phillippi, M. A. & Mott, J. L. XIAP Antagonist embelin inhibited proliferation of cholangiocarcinoma cells. PLoS ONE 9, e90238 (2014).
Mertens, J. C., Fingas, C. D., Christensen, J. D., Smoot, R. L., Bronk, S. F., Werneburg, N. W. et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 73, 897–907 (2013).
Sirica, A. E. & Gores, G. J. Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology 59, 2397–2402 (2014).
Carpino, G., Overi, D., Melandro, F., Grimaldi, A., Cardinale, V., Di Matteo, S. et al. Matrisome analysis of intrahepatic cholangiocarcinoma unveils a peculiar cancer-associated extracellular matrix structure. Clin. Proteomics 16, 37 (2019).
Whiteside, T. L. What are regulatory T cells (Treg) regulating in cancer and why? Semin. Cancer Biol. 22, 327–334 (2012).
Ghidini, M., Cascione, L., Carotenuto, P., Lampis, A., Trevisani, F., Previdi, M. C. et al. Characterisation of the immune-related transcriptome in resected biliary tract cancers. Eur. J. Cancer 86, 158–165 (2017).
Verlingue, L., Malka, D., Allorant, A., Massard, C., Ferté, C., Lacroix, L. et al. Precision medicine for patients with advanced biliary tract cancers: an effective strategy within the prospective MOSCATO-01 trial. Eur. J. Cancer 87, 122–130 (2017).
Sicklick, J. K., Kato, S., Okamura, R., Schwaederle, M., Hahn, M. E., Williams, C. B. et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat. Med. 25, 744–750 (2019).
Mody, K., Kasi, P. M., Yang, J., Surapaneni, P. K., Bekaii-Saab, T., Ahn, D. H., et al. Circulating tumor DNA profiling of advanced biliary tract cancers. JCO Precis. Oncol. https://doi.org/10.1200/PO.18.00324 (2019).
Shen, N., Zhang, D., Yin, L., Qiu, Y., Liu, J., Yu, W. et al. Bile cell‑free DNA as a novel and powerful liquid biopsy for detecting somatic variants in biliary tract cancer. Oncol. Rep. 42, 549–560 (2019).
Yang, J. D., Campion, M. B., Liu, M. C., Chaiteerakij, R., Giama, N. H., Ahmed Mohammed, H. et al. Circulating tumor cells are associated with poor overall survival in patients with cholangiocarcinoma. Hepatology 63, 148–158 (2016).
Vlachogiannis, G., Hedayat, S., Vatsiou, A., Jamin, Y., Fernández-Mateos, J., Khan, K. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926 (2018).
Amato, F., Rae, C., Prete, M. G. & Braconi, C. Cholangiocarcinoma disease modelling through patients derived organoids. Cells 9, 832 (2020).
Acknowledgements
This work was performed under the frame of Working Group 6 of the COST Action collaboration (COST Action CA18122 European Cholangiocarcinoma Network, EURO-CHOLANGIO-NET).
Author information
Authors and Affiliations
Consortia
Contributions
C.B., J.J.G.M., M.G.P., A.L., S.T., A.L.M., G.B., O.S., A.V., R.I.R.M., P.M.R., A.C., M.J., C.M.P.R., M.G.F.-B., A.S.R., M.M., G.M., P.A., P.M.G., V.C., J.M.B., J.W.V. and J.B. contributed to the writing and the review of the paper. All authors approved the final version of the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
Not applicable.
Competing interests
The authors declare the following competing interest: C.B. and/or her family members received speaker honoraria from Bayer, EliLilly, Pfizer and Merck-Serono. A.L.M. received travel funding from Pfizer, Merck, Roche and Lilly. A.L. received travel and educational support from Ipsen, Pfizer, Bayer, AAA, SirtEx, Novartis, Mylan and Delcath; speaker honoraria from Merck, Pfizer, Ipsen, Incyte and AAA; advisory honoraria from EISAI, Nutricia Ipsen, QED and Roche; she is also a member of the Knowledge Network and NETConnect Initiatives funded by Ipsen. A.V. received honoraria form Meck, MSD, BMS, INCYTE, AstraZeneca, Roche, Bayer, Basilea, BTG, Novartis and Decath. J.W.V. received honoraria from Agios, AstraZeneca, Debiopharm, Delcath Systems, GenoScience Pharma, Imaging Equipment Ltd, Incyte, Ipsen, Keocyt, Merck, Mundipharma EDO, Novartis, Nucana, PCI Biotech, Pieris Pharmaceuticals, Pfizer, QED, Servier and Wren Laboratories, and declares Speakers’ Bureau for Imaging Equipment Ltd, Ipsen, Novartis and Nucana.
Funding information
C.B. LKAS readership—University of Glasgow: J.J.G.M./R.I.R.M. Carlos III Institute of Health, Spain (PI16/00598); “Centro Internacional sobre el Envejecimiento”, Spain (OLD-HEPAMARKER, 0348-CIE-6-E); C.M.P.R. is supported by FEDER funds through the COMPETE programme and by national funds through Fundação para a Ciência e a Tecnologia (grants PTDC/MED-FAR/29097/2017 and SAICTPAC/0019/2015—LISBOA-01-0145-FEDER-016405). A.L. was part-funded by The Christie Charity. MGF-B is supported by grants SAF2014-54191-R and SAF2017-88933-R from FEDER/Ministerio de Ciencia, Innovación y Universidades-Agencia Estatal de Investigación; HEPACARE Project from Fundación La Caixa. J.M.B. was funded by Spanish Carlos III Health Institute (ISCIII) (FIS PI15/01132, PI18/01075 and Miguel Servet Program CON14/00129), cofinanced by “Fondo Europeo de Desarrollo Regional” (FEDER); AMMF (J.M. Banales and P.M. Rodrigues 2019/202); PSC Partners US; PSC Supports UK (06119JB); Horizon 2020 (H2020 ESCALON project: H2020-SC1-BHC-2018-2020); IKERBASQUE, Basque foundation for Science; CIBERehd (ISCIII); “Diputación Foral Gipuzkoa” (DFG15/010, DFG16/004), BIOEF (Basque Foundation for Innovation and Health Research); EiTB Maratoia (BIO15/CA/016/BD); Department of Health of the Basque Country (2017111010) and Euskadi RIS3 (2016222001, 2017222014, 2018222029 and 2019222054); La Caixa Scientific Foundation (HR17-00601); “Fundación Científica de la Asociación Española Contra el Cáncer” (AECC Scientific Foundation, to J.M. Banales and J.J.G. Marin). P.M.R. was funded by Spanish Carlos III Health Institute (ISCIII; Sara Borrell CD19/00254) cofinanced by “Fondo Europeo de Desarrollo Regional”. M.M. received PSA-2017-UNIVPM. P.A. is supported by a NIHR-Biomedical Research Centre funding (BRC646b/III/SP/101350). O.S. is funded by AIRC (IG2018, ID 21627, PI Segatto Oreste). A.V. is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - SFB/TRR 209 - 314905040, and Vo959/9-1. This work was performed under the frame of Working Group 6 of the COST Action CA18122 European Cholangiocarcinoma Network supported by COST (European Cooperation in Science and Technology) http://www.cost.eu.
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the authors have noticed that acknowledgement of the support given by COST (European Cooperation in Science and Technology) to the study was omitted from the Funding section.
Rights and permissions
About this article
Cite this article
Marin, J.J.G., Prete, M.G., Lamarca, A. et al. Current and novel therapeutic opportunities for systemic therapy in biliary cancer. Br J Cancer 123, 1047–1059 (2020). https://doi.org/10.1038/s41416-020-0987-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-0987-3
This article is cited by
-
Macro CD5L+ deteriorates CD8+T cells exhaustion and impairs combination of Gemcitabine-Oxaliplatin-Lenvatinib-anti-PD1 therapy in intrahepatic cholangiocarcinoma
Nature Communications (2024)
-
Sintilimab plus nab-paclitaxel as second-line treatment for advanced biliary tract cancer: study protocol for an investigator-initiated phase 2 trial (NapaSinti trial)
BMC Cancer (2023)
-
Prognostic value of Dickkopf-1 and ß-catenin expression according to the antitumor immunity of CD8-positive tumor-infiltrating lymphocytes in biliary tract cancer
Scientific Reports (2022)