Abstract
The coronavirus disease 2019 (COVID-19) pandemic epicentre has moved to the USA and Europe, where it is placing unprecedented demands on healthcare resources and staff availability. These service constraints, coupled with concerns relating to an increased incidence and severity of COVID-19 among patients with cancer, should lead to re-consideration of the risk–benefit balance for standard treatment pathways. This is of particular importance to pancreatic cancer, given that standard diagnostic modalities such as endoscopy may be restricted, and that disease biology precludes significant delays in treatment. In light of this, we sought consensus from UK clinicians with an interest in pancreatic cancer for management approaches that would minimise patient risk and accommodate for healthcare service restrictions. The outcomes are described here and include recommendations for treatment prioritisation, strategies to bridge to later surgical resection in resectable disease and factors that modify the risk–benefit balance for treatment in the resectable through to the metastatic settings. Priority is given to strategies that limit hospital visits, including through the use of hypofractionated precision radiotherapy and chemoradiotherapy treatment approaches.
Similar content being viewed by others
Background
Following the first reports of infection with severe acute respiratory syndrome coronavirus 2 during December 2019 in Wuhan, China, cases of coronavirus disease 2019 (COVID-19) have dramatically increased across the world.1 With its epicentre now in Europe and the USA, the COVID-19 pandemic is placing unprecedented demands on healthcare resources across a number of countries. This includes the United Kingdom (UK), where increasing numbers of patients critically unwell from COVID-19 have in some areas severely diminished bed availability within high-dependency and intensive care units, reducing surgical capacity as a consequence. A reduction in the numbers of frontline healthcare workers through infection and self-isolation is also increasing service pressures.
Adding further challenge to standard cancer treatment pathways, a majority of patients with cancer are immunosuppressed and may be more likely to contract COVID-19.2,3,4,5,6 Given that hospitals act as a reservoir for infection, this risk is amplified by multiple hospital attendances for cancer diagnosis, treatment and follow-up. A cancer diagnosis and recent anticancer treatment may additionally be linked to greater severity of COVID-19.2,3,4,5,6 As such, the risk–benefit balance is likely to have changed for a number of cancer treatments, although it should be noted that evidence of the magnitude of risk conferred by COVID-19 for patients with cancer, and for those receiving anticancer therapies, remains uncertain.7 Adding further complexity, cancer services must now forward plan for possible recurrent peaks in COVID-19 incidence while managing the lasting consequences of the first outbreak. This includes both a backlog of cases resulting from the clear pivot of the National Health Service (NHS) towards a focus on COVID-19 treatment and, in some areas, continuing to grapple with a prolonged plateau in first peak COVID-19 incidence.
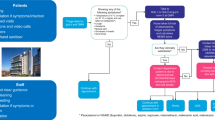
In light of this, we convened an expert group of UK clinicians with expertise in pancreatic cancer. The panel identified areas in which resource limitations or the potential for SARS-CoV-2 infection would potentially increase the risks of, or limit access to, current standard treatments for pancreatic cancer. This included a review of guidance relating to COVID-19 published by NHS England and other relevant UK professional bodies. Alternative management strategies for these scenarios were sought via literature review and through input from panel members. Identified options were virtually reviewed by the panel and used to formulate an initial guidance document. This subsequently received iterative input from the panel until consensus was reached, with a focus throughout on management approaches that would minimise risk to the patient and accommodate for healthcare service restrictions, such as through where possible limiting hospital attendance in line with the RADS (Remote, Avoid, Defer, Shorten) principle.8,9 The 18-member panel, which included surgeons, clinical (radiation) oncologists and medical oncologists, are listed in Supplementary information. Additional feedback was received from patient and public representatives via Pancreatic Cancer UK, a registered pancreatic cancer charity.
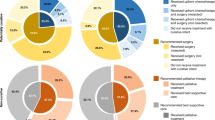
The proposals developed through this process are summarised in Table 1 and have been revised as the COVID-19 outbreak has evolved. They should serve to guide clinicians both as the initial COVID-19 peak plateaus and resolves, and in any subsequent disease outbreaks. These should be considered in conjunction with other documents outlining stratification and prioritisation of surgery, chemotherapy and radiotherapy (RT) delivery during the COVID-19 pandemic.10,11,12,13,14,15
Diagnosis of pancreatic cancer
With a number of other stakeholders, the British Society of Gastroenterology has published guidance categorising upper gastrointestinal endoscopy as an aerosol-generating procedure and recommending that all elective and non-essential endoscopic procedures should stop.16 It is recommended that endoscopic therapy should continue for malignant biliary obstruction, providing an opportunity to retrieve cytology from biliary strictures or in the case of peri-ampullary neoplasm biopsy specimens for some patients prior to self-expanding metal stent insertion. In contrast, 2-week wait cancer referrals and cancer staging endoscopic ultrasound are to be discussed on a case-by-case basis. In instances where histology or cytology cannot be obtained, the multidisciplinary team (MDT) should reach a treatment recommendation based on balancing the risks of inappropriately treating an alternative pathology, such as chronic or autoimmune pancreatitis, against a watch-and-wait approach. Options include proceeding to definitive treatment based on imaging and elevated tumour markers where there is strong suspicion of malignancy or offering treatment where repeat investigations provide evidence for disease progression. Where there is diagnostic uncertainty, patients must be counselled regarding the possibility that they might not have cancer, but would be at risk of developing life-threatening treatment complications, or that in the absence of knowledge of the histological cancer subtype, their treatment might be suboptimal. Percutaneous biopsy may be feasible for more advanced disease, while percutaneous fine-needle aspiration may also have to be considered for localised disease if supported by radiology and pathology expertise.
Treatment by disease stage
General principles
There is emerging but relatively low-level evidence that COVID-19 confers additional risk for patients with cancer, although this is not as yet robustly quantified.7 Strategies to manage pancreatic cancer should balance this risk and the impact of healthcare resource limitations against the potential benefits of treatment; not least given that significant delays in therapy would ordinarily be precluded by disease biology.17,18 Selected approaches will need to adapt to emerging evidence related to COVID-19 and to changes in the availability of key resources. Based on guidance and priority setting from NHS England and the National Institute for Health and Clinical Excellence, systemic anticancer therapy for patients with resectable disease (priority levels 2–4) should be ranked over locally advanced pancreatic cancer (LAPC; priority levels 4 and 5) and metastatic disease (priority levels 4–6), should prioritisation be required (see Supplementary Table 1).9,11 A balanced discussion with patients is required to contextualise the known and potential risks of COVID-19 against both the risks of complications from the cancer itself and the potential for complications from anticancer therapy and potential resource limitations. In particular, it must be highlighted that our current ability to mitigate and manage complications associated with pancreatic surgery is predicated on an unlimited access to multidisciplinary services, including physiotherapy, dietetics, nursing, interventional radiology and intensive care.
Where SACT is administered, pragmatic options to mitigate risk include dose modification and the use of prophylactic growth factors and antibiotics. It is also important that all patients adhere to the principles of physical distancing and that they are supported to do so, such as through the use of telephone consultations and remote assessments. In addition, clinical trials and technical development initiatives (robotic surgery) should be stopped in order to minimise resource burden.
In the event of varying regional pressures, particularly during any second peaks of COVID-19, it may be beneficial to refer patients for management in other regions. Where possible, this option should be pursued and facilitated in order to ensure that regional resource limitations do not hinder the provision of optimal care.
Resectable and borderline resectable disease
Options for upfront resection are likely to be severely limited at the initial height of the COVID-19 pandemic or in the event of recurrent peaks in incidence. Consolidation of surgery in ‘ringfenced’ clean sites has helped to support some surgical capacity during the first COVID-19 peak, although these centres have limited capacity and are likely to be highly selective. Surgery for resectable pancreatic cancer remains the optimal standard of care, and where available should be pursued. Cancer presentation, patient comorbidity, disease severity, regional pandemic burden and regional hospital resources should be considered when selecting patients for surgery. These decisions are likely to remain dynamic and should draw on recommendations from SAGES-AHPBA (Society of American Gastrointestinal and Endoscopic Surgeons-American Hepato-Pancreato-Biliary Association).19
Where surgery is unlikely to be available due to a lack of capacity or resources, consider upfront chemotherapy and/or chemoradiotherapy (CRT). Treatment options include SACT (evidence level 2a) and hypofractionated precision RT/CRT, as outlined below, following an informed consent process.20 For RT consider a dose of 25–35 Gy/5 fractions (RT alone, dose depending on centre expertise) (evidence level 4) or 36 Gy/15 fractions CRT with concurrent capecitabine (evidence level 1b).21,22 For SACT, a combination of 5-fluorouracil, folinic acid, irinotecan and oxaliplatin (FOLFIRINOX) is preferred as the reported median progression-free interval of 15 months could allow deferral of resection in selected patients.23 While the magnitude of the additional increase risk conferred by COVID-19 to patients with cancer, particularly those undergoing chemotherapy, is unclear, the risk of death is significantly greater in those with comorbidities and those over 70 years of age.24,25 As such, FOLFIRINOX may be most appropriate in patients with a good performance status without significant comorbidities.
Decisions relating to the administration of adjuvant chemotherapy should take into account patient choice, followed by counselling of its risks and benefits. In the absence of adjuvant chemotherapy, 5-year survival for patients who have undergone resection is <10%, compared with over 20% for those who receive adjuvant treatment.26,27,28,29 For example, in a recent randomised controlled trial, adjuvant FOLFIRINOX delivered 3-year disease-free survival of 39.7% and median overall survival of 54.4 months.26 Treatment could also be deferred for up to 12 weeks from surgery (evidence level 1b).30 As with neoadjuvant SACT, decision on appropriateness and choice of regimen should be guided by age, comorbidity and potential magnitude of benefit. Nodal status should also be considered given evidence that the outcomes of patients without nodal metastases is more favourable.28 The increased effectiveness of combination chemotherapy needs to be balanced with the increased risks of complications, including those relating to COVID-19.
Locally advanced pancreatic cancer
Patients with LAPC are conventionally managed with upfront chemotherapy, with or without consolidation CRT. The use of upfront hypofractionated (5 fractions, evidence level 2a) or, alternatively, 15 fractions CRT (evidence level 4) may provide lower-risk alternatives and may allow delaying the initiation of or a break in SACT (evidence level 2a).31 This approach should, however, be weighed against the risk of early metastatic progression without upfront chemotherapy.30 Given the increasing risks of COVID-19 with age, the risks of treatment in those aged over 80 years are likely to outweigh any benefit and no intervention is likely to be the best option for the majority of patients. For fit patients without significant comorbidities, consider four cycles of modified FOLFIRINOX with or without consolidation hypofractionated CRT or five fraction RT alone23,32 (evidence level 2a).
Metastatic disease
The risks of treatment for metastatic disease are likely to outweigh the benefits in many patients as the median improvement in survival is usually <6 months. A decision to initiate palliative chemotherapy should be individualised and highly selective; options for consideration include single-agent gemcitabine, gemcitabine plus nab-paclitaxel and FOLFIRINOX in order of increasing efficacy and increasing toxicity (evidence level 1b).33,34 In order to mitigate risks, clinicians should consider early response assessment (if radiology capacity allows) to limit duration of chemotherapy. A break from chemotherapy may be considered in patients with low volume disease or those with good disease control (evidence level 5). The limited benefits of second-line chemotherapy outweigh the potential benefits and should not be routinely offered to patients (evidence level 5).
Hypofractionated radiation approaches
Frequent hospital visits will increase risk of patients contracting COVID-19, therefore conventional CRT (25–30 fractions) should be avoided. Hypofractionated RT (5–15 fractions) reduces footfall, is less immunosuppressive than chemotherapy and the total overall time in hospital is likely to be less than or comparable to patients receiving 3 months of FOLFIRINOX-or gemcitabine-based chemotherapy. Detailed RT delivery guidance document and evidence for their use is available at www.uppergicancer.com. A summary of key points is provided in Table 2.
RT alone
Dose fractionation: 30 Gy/5 fractions (range 25–35 Gy/5 fractions, daily or alternate day fractionation)
Oncologists who have experience of delivering upper abdominal/pancreatic stereotactic ablative radiotherapy (SABR) could deliver radiation at higher doses of 33–35 Gy/5 fractions using SABR. For those without this expertise, a lower dose of 30 Gy/5 fraction should be considered. Simultaneous integrated boost to tumour/vessel contact (40 Gy) may be considered.35
Chemoradiotherapy
Dose fractionation: 36 Gy/15 (preoperative CRT) or 45 Gy/15 fractions (definitive CRT) with capecitabine (830 mg/m2 b.d. on days of RT)
This regime should be deliverable by all units with experience in pancreatic RT, the final doses being driven by the normal tissue constraints. A dose of 45–50 Gy/15 fractions is radiobiologically equivalent to conventionally fractionated regimes used in the UK. While the α/β value for pancreatic adenocarcinoma has not been fully elucidated, it is likely to range between 4 and 10, giving an EQD2 (equivalent dose) of 52.5–61.6 Gy, assuming an α/β of 4, or of 48.8–55.6 Gy, assuming an α/β of 10.36,37
Summary
The COVID-19 pandemic poses an unprecedented challenge to the management of patients with cancer; both through a heightened risk of life-threatening infection and through pressure on health services. We have outlined here, based on the best available evidence and UK expert consensus, suggestions for optimising the outcomes of patients with pancreatic cancer. It is vital that decisions are individualised for patients following MDT discussion, and that patients are comprehensively counselled regarding treatment options prior to providing informed consent. Equally, it will be important to evaluate the management options outlined here and clinicians are encouraged to visit www.uppergicancer.com to participate in prospective data collection. Finally, while there is a need to accommodate for the enhanced risks and impact on services from COVID-19, this must not result in a return to the nihilism that has dogged pancreatic cancer for many decades. In these challenging times, compassion and empathy remain key during what is already a frightening period for our patients.
References
Li, Q., Guan, X., Wu, P., Wang, X., Zhou, L., Tong, Y. et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 382, 1199–1207 (2020).
Yu, J., Ouyang, W., Chua, M. L. K. & Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2020.0980 (2020).
Liang, W., Guan, W., Chen, R., Wang, W., Li, J., Xu, K. et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 21, 335–337 (2020).
Xia, Y., Jin, R., Zhao, J., Li, W. & Shen, H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 21, e180 (2020).
Guan, W., Liang, W., Zhao, Y., Liang, H., Chen, Z., Li, Y. et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur. Resp. J. 55, 2000547 (2020).
Miyashita, H., Mikami, T., Chopra, N., Yamada, T., Chernyavsky, S., Rizk, D. et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann. Oncol. S0923-7534, 39303–0 (2020).
Robinson, A. G., Gyawali, B. & Evans, G. COVID-19 and cancer: do we really know what we think we know? Nat. Rev. Clin. Oncol. 18, 1–3 (2020).
Zaorsky, N. G., Yu, J. B., McBride, S. M., Dess, R. T., Jackson, W. C. & Mahal B. A. et al. Prostate cancer radiotherapy recommendations in response to COVID-19. Adv. Radiat. Oncol. https://doi.org/10.1016/j.adro.2020.03.010 (2020).
National Institute for Health & Care Excellence (NICE). COVID-19 rapid guideline: delivery of radiotherapy. NICE Guideline [NG162] https://www.nice.org.uk/guidance/NG162 (2020).
Filippi, A. R., Russi, E., Magrini, S. M. & Corvo, R. Letter from Italy: first practical indications for radiation therapy departments during COVID-19 outbreak. Int. J. Radiat. Oncol. Biol. Phys. S0360–3016, 30930–30935 (2020).
NHS England. Clinical guide for the management of cancer patients during the coronavirus pandemic. NHS England Version. 1 https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-acute-treatment-cancer-23-march-2020.pdf (2020).
Simcock, R., Thomas, T. V., Mercy, C. E. et al. COVID-19: global radiation oncology’s targeted response for pandemic preparedness. Clin. Transl. Radiat. Oncol. 22, 55–68 (2020).
National Institute for Health and Care Excellence (NICE). COVID-19 rapid guideline: delivery of systemic anticancer treatments. NICE guideline [NG161] https://www.nice.org.uk/guidance/ng161 (2020).
American College of Surgeons. COVID-19: recommendations of management of elective surgical procedures. https://www.facs.org/covid-19/clinical-guidance/elective-surgery (2020).
Royal College of Surgeons. Intercollegiate general surgery guidance on COVID-19. https://www.rcsed.ac.uk/news-public-affairs/news/2020/march/intercollegiate-general-surgery-guidance-on-covid-19-update (2020).
British Society of Gastroenterology. Endoscopy activity and COVID-19: BSG and JAG guidance. https://www.bsg.org.uk/covid-19-advice/endoscopy-activity-and-covid-19-bsg-and-jag-guidance/ (2020).
Ahn, S. J., Choi, S. J. & Kim, H. S. Time to progression of pancreatic cancer: evaluation with multi-detector computed tomography. J. Gastrointest. Cancer 48, 164–169 (2017).
Yu, J., Blackford, A. L., Dal Molin, M., Wolfgang, C. L. & Goggins, M. Time to progression of pancreatic ductal adenocarcinoma from low-to-high tumour stages. Gut 64, 1783–1789 (2015).
Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) & The Americas Hepato-Pancreato-Biliary Association (AHPBA). https://www.sages.org/sages-ahpba-recommendations-surgical-management-of-hpb-cancer-covid-19/ (2020).
Zhan, H. X., Xu, J. W., Wu, D., Wu, Z. Y., Wang, L., Hu, S. Y. et al. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med. 6, 1201–1219 (2017).
Xiang, M., Heestand, G. M., Chang, D. T. & Pollom, E. L. Neoadjuvant treatment strategies for resectable pancreas cancer: a propensity-matched analysis of the National Cancer Database. Radiother. Oncol. 143, 101–107 (2020).
Versteijne, E., Suker, M., Groothuis, K., Akkermans-Vogelaar, J. M., Besselink, M. G., Bonsing, B. A. et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J. Clin. Oncol. https://doi.org/10.1200/JCO.19.02274 (2020).
Suker, M., Beumer, B. R., Sadot, E., Marthey, L., Faris, J. E., Mellon, E. A. et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 17, 801–810 (2016).
Weiss, P. & Murdoch, D. R. Clinical course and mortality risk of severe COVID-19. Lancet 395, 1014–1015 (2020).
Verity, R., Okell, L. C., Dorigatti, I., Winskill, P., Whittaker, C., Imai, N. et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect. Dis. S1473-3099, 30243–30247 (2020).
Neoptolemos, J. P., Stocken, D. D., Friess, H., Bassi, C., Dunn, J. A., Hickey, H. et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 350, 1200–1210 (2004).
Oettle, H., Neuhaus, P., Hochhaus, A., Hartmann, J. T., Gellert, K., Ridwelski, K. et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 310, 1473–1481 (2013).
Neoptolemos, J. P., Palmer, D. H., Ghaneh, P., Psarelli, E. E., Valle, J. W., Halloran, C. M. et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 389, 1011–1024 (2017).
Conroy, T., Hammel, P., Hebbar, M., Abdelghani, M. B., Wei, A. C., Raoul, J.-L. et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 379, 2395–2406 (2018).
Valle, J. W., Palmer, D., Jackson, R., Cox, T., Neoptolemos, J. P., Ghaneh, P. et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J. Clin. Oncol. 32, 504–512 (2014).
Petrelli, F., Comito, T., Ghidini, A., Torri, V., Scorsetti, M. & Barni, S. Stereotactic body radiation therapy for locally advanced pancreatic cancer: a systematic review and pooled analysis of 19 trials. Int. J. Radiat. Oncol. Biol. Phys. 97, 313–322 (2017).
Tchelebi, L. T., Lehrer, E. J., Trifiletti, D. M., Sharma, N. K., Gusani, N. J., Crane, C. H. et al. Conventionally fractionated radiation therapy versus stereotactic body radiation therapy for locally advanced pancreatic cancer (CriSP); an international systematic review and meta-analysis. Cancer 126, 2120–2131 (2020).
Conroy, T., Desseigne, F., Ychou, M., Bouche, O., Guimbaud, R., Becouarn, Y. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825 (2011).
Von Hoff, D. D., Ervin, T., Arena, F. P., Chiorean, E. G., Infante, J., Moore, M. et al. Increased survival in pancreatic cancer with nab-Paclitaxel plus gemcitabine. N. Engl. J. Med. 369, 1691–1703 (2013).
Holyoake, D. L. P., Ward, E., Grose, D., McIntosh, D., Sebag-Montefiore, D., Radhakrishna, G. et al. A phase-I trial of pre-operative, margin intensive, stereotactic body radiation therapy for pancreatic cancer: the ‘SPARC’ trial protocol. BMC Cancer 16, 728 (2016).
Prior Jr., P. W., Chen, X., Hall, W. A., Erickson, B. A. & Li A. Estimation of the alpha–beta ratio for chemoradiation of locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. 102, https://doi.org/10.1016/jijrobp.2018.06.250 (2018).
Jones, B., Dale, R. G. & Hopewell, J. Additional guidance on management of unscheduled radiotherapy treatment interruptions in patients during the COVID-19 pandemic. https://www.rcr.ac.uk/sites/default/files/cancer-treatment-gaps-covid19.pdf (2020).
Author information
Authors and Affiliations
Contributions
All authors contributed to the development of the consensus guidance provided here. S.M. led the process to develop this guidance and C.M.J. authored the first draft of the manuscript. All authors contributed to subsequent revisions of the manuscript. G.R., K.A., R.G., J.G., D.G., D.H., A.H., M.A.H. and S.M. contributed to the development of the pancreas radiation protocols.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Competing interests
The authors declare that they have no relevant conflicts of interest. J.W.V. reports personal fees from AstraZeneca, personal fees from Debiopharm, personal fees from Delcath Sytems, personal fees from Genoscience Pharma, personal fees from Imaging Equipment Limited, personal fees from Incyte, personal fees from Ipsen, personal fees from Keocyt, personal fees from Merck, personal fees from Mundipharma EDO, personal fees from Novartis, grants, personal fees and non-financial support from NuCana, personal fees from PCI Biotech, personal fees from Pieris Pharmaceuticals, personal fees and non-financial support from Pfizer, personal fees from QED, grants and personal fees from Servier, personal fees from Wren Laboratories and personal fees from Agios, all outside the submitted work. S.M. has received research funding from Celgene.
Funding information
C.M.J. is supported by a Wellcome Trust Clinical Research Fellowship. K.A. and A.H. acknowledge NHS funding to the NIHR Biomedical Research Centre at The Royal Marsden and the Institute of Cancer Research. N.B.J. is supported by a Cancer research UK Clinician Scientist Fellowship (C55370/A25813). M.A.H. is supported by funding from the NIHR Biomedical Research Centre at University College London Hospitals NHS Foundation Trust and University College London. S.M. is supported by funding from the NIHR Oxford Biomedical Research Centre.
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jones, C.M., Radhakrishna, G., Aitken, K. et al. Considerations for the treatment of pancreatic cancer during the COVID-19 pandemic: the UK consensus position. Br J Cancer 123, 709–713 (2020). https://doi.org/10.1038/s41416-020-0980-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-0980-x
This article is cited by
-
The impact of the COVID-19 pandemic upon pancreatic cancer treatment (CONTACT Study): a UK national observational cohort study
British Journal of Cancer (2023)
-
Neoadjuvant therapy for pancreatic cancer
Updates in Surgery (2022)
-
Reply to Comment on “The UK consensus position on the treatment of pancreatic cancer during the COVID-19 pandemic”
British Journal of Cancer (2021)
-
Comment on “Considerations for the treatment of pancreatic cancer during the COVID-19 pandemic: the UK consensus position”
British Journal of Cancer (2021)
-
Cancer or COVID-19? A Review of Guidelines for Safe Cancer Care in the Wake of the Pandemic
SN Comprehensive Clinical Medicine (2020)