Abstract
Background
The relationship between long-chain omega-3 (LCn3), alpha-linolenic acid (ALA), omega-6 and total polyunsaturated fatty acid (PUFA) intakes and cancer risk is unclear.
Methods
We searched Medline, Embase, CENTRAL and trials registries for RCTs comparing higher with lower LCn3, ALA, omega-6 and/or total PUFA, that assessed cancers over ≥12 months. Random-effects meta-analyses, sensitivity analyses, subgrouping, risk of bias and GRADE were used.
Results
We included 47 RCTs (108,194 participants). Increasing LCn3 has little or no effect on cancer diagnosis (RR1.02, 95% CI 0.98–1.07), cancer death (RR0.97, 95% CI 0.90–1.06) or breast cancer diagnosis (RR1.03, 95% CI 0.89–1.20); increasing ALA has little or no effect on cancer death (all high/moderate-quality evidence). Increasing LCn3 (NNTH 334, RR1.10, 95% CI 0.97–1.24) and ALA (NNTH 334, RR1.30, 95% CI 0.72–2.32) may slightly increase prostate cancer risk; increasing total PUFA may slightly increase risk of cancer diagnosis (NNTH 125, RR1.19, 95% CI 0.99–1.42) and cancer death (NNTH 500, RR1.10, 95% CI 0.48–2.49) but total PUFA doses were very high in some trials.
Conclusions
The most extensive systematic review to assess the effects of increasing PUFAs on cancer risk found increasing total PUFA may very slightly increase cancer risk, offset by small protective effects on cardiovascular diseases.
Similar content being viewed by others
Background
Cancer is a leading cause of morbidity and mortality worldwide with ~17 million new cases and 9.6 million cancer-related deaths in 2018.1 The most common cancers worldwide are lung, female breast, bowel and prostate cancer, accounting for 40% of cancers diagnosed.1 23% of UK breast cancer cases are thought to be preventable, with causes including overweight and obesity (8%), alcohol (8%), not breastfeeding (5%), post-menopausal hormones (2%) and oral contraceptives (<1%).1 Preventability appears to vary so 79% of lung cancer cases are preventable (and mainly due to smoking), 54% of bowel cancer (causes including too little dietary fibre, processed meat, overweight and obesity, alcohol, smoking and sedentary behaviour) and an unknown proportion of prostate cancer (risk factors are unclear).1 Every sixth death in the world is due to cancer2 and in the USA cancer expenditure is projected as $156 billion by 2020,3 so even small beneficial or harmful effects could be important. The other major health risk worldwide is cardiovascular disease, responsible for 37% of premature deaths due to non-communicable disease in 2012, where cancers were responsible for 27%.4
Dietary polyunsaturated fatty acids (PUFA) have been postulated as a modifiable component of lifestyle that could influence cancer risk. PUFA includes long-chain omega-3 (LCn3 including eicosapentaenoic acid and docosapentaenoic acid), alpha-linolenic acid (ALA, a shorter chain omega-3) and omega-6 fats (including linoleic acid, LA). Polyunsaturated fats are common healthy eating choices, and fish oil (LCn3) and flaxseed (ALA) supplements commonly consumed.5 Potential mechanisms for PUFAs in cancer aetiology include their being precursors to lipid mediators regulating metabolic pathways and inflammatory responses,6 oxidative stress, and changes in membrane composition that could affect cell signalling pathways.7 Reducing dietary fat (including PUFAs) appears to result in lower weight in adults,8 so lower PUFA intake (as part of general fat reduction) could offer protective effects against those cancers that are associated with overweight. These mechanisms suggest that omega-3 may be protective, and omega-6 and total PUFA may exacerbate cancer risk. However, oily fish and fish oil capsules may contain contaminants such as mercury and dioxins, potential carcinogens.9,10,11,12
Evidence for effects of polyunsaturated fats on risk of cancer is conflicting. An early RCT, the Lyon Diet Heart Study, suggested that a Mediterranean type diet, supplemented with an experimental canola (rapeseed) oil-based margarine rich in oleic and ALA, reduced cancer diagnoses by 61% compared to those on the American Heart Association diet.13 Within the Japanese population, whose traditional diet is rich in oily fish, incidence of some cancers has increased with more westernised food consumption and lifestyles.6 One systematic review of cohort studies did not pool data but found some cohorts with positive associations, some with negative associations and more with null associations for omega-3 and a variety of cancers, including breast and prostate cancer – overall there was no trend to suggest that omega-3 fatty acids are associated with total cancer risk.14 A systematic review of 10 RCTs comparing high to low omega-3 intake for at least 6 months found no evidence that increasing omega-3 fats altered cancer incidence.15 Later meta-analysis of RCTs increasing omega 3 intake over at least 6 months found omega-3 supplementation increased the risk of cancer by 10% but this was not statistically significant,16 and this review did not analyse for specific cancer types, provided limited information on dosage and did not stratify by supplementation level.
The Mediterranean diet, which is high in polyunsaturated fats, has attracted attention because of the historically lower breast cancer rates in Mediterranean countries than in other parts of Europe and the United States.17,18 A cohort study of over 35,000 post-menopausal US women suggested that taking omega-3 supplements was associated with a 32% reduction in breast cancer risk,19 although other cohort studies are not consistent in this relationship.20 A large European cohort study (EPIC) found no association between fatty fish consumption and breast cancer risk.21 Comprehensive systematic reviews of observational studies suggested no relationship between total polyunsaturated fat intake and risk of breast cancer22 or omega-3 intake and breast cancer risk.23
Two nested case-control studies of men suggested that high serum long-chain omega-3 fatty acids were associated with increased risk of prostate cancer and high-grade prostate cancer,24,25 but a systematic review found inadequate data to determine whether fish-derived omega-3 fatty acids were associated with prostate cancer incidence and progression.26
Some polyunsaturated fats are essential in the human diet, and UK dietary reference values suggest we need to eat at least 6.5% of our energy intake in the form of cis-polyunsaturated fats.27 Further increasing polyunsaturated fat intake is associated with healthy eating and prevention of cardiovascular disease in the general public, but is still scientifically controversial.28 The use of supplements as additions or replacements to food stuff has gained traction with the general public. It has been estimated that approximately 38% of American adults use complementary medicines and fish oil, omega 3 or DHA supplements are the most commonly used non-vitamin, non-mineral natural product (37.4%) and flaxseed the 4th (15.9%).5 LCn3 is ingested in the form of oily fish or fish oil (often fish liver oil) capsules, however, these may contain contaminants. Heavy metals such as mercury, cadmium, chromium, nickel, lead and cobalt and toxic compounds such as dioxins have been found in fish and fish oils representing a potential risk to health.9,10,11,12 It is therefore important to assess both potential benefits and harms of increasing omega-3, omega-6 and total polyunsaturated fats on cancer risk to better inform members of the public considering dietary change or supplementation.
As previous systematic reviews of trials and observational studies have been equivocal about effects of omega-3, omega-6 and total PUFA on total, breast and prostate cancer risk,15,16,22,23,26,29,30,31,32 this review assessed the risks and protective effects of increasing omega 3, omega 6 and total polyunsaturated fat (PUFA) intake on total, breast and prostate cancer incidence in adults, gathering a much larger set of randomised trials than has previously been assessed as it included trials where cancer diagnosis was not the primary outcome, but cancer diagnosis or mortality data were available. As this systematic review was conducted as part of a series of systematic reviews assessing a range of health effects of omega-3, omega-6 and total PUFA33,34,35,36,37,38 (Ajabnoor et al., personal communication, Brainard et al., personal communication) we have been able to compare health benefits and harms across the major causes of mortality and morbidity in developed countries: cancer and cardiovascular disease.
Methods
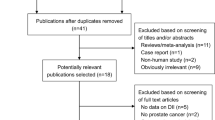
Methods for the series have been reported in detail (including the PRISMA flow diagram and detailed search strategies).39 This review’s protocol was registered on PROSPERO40 and its specific methods are summarised below.
Inclusion criteria
We included randomised controlled trials (RCTs) that compared higher versus lower LCn3, ALA, omega-6 and/or total PUFA in adults aged at least 18 years, who were not pregnant or seriously ill. Participants could be free of cancer, at increased risk of cancer or with a previous cancer diagnosis, but were excluded if they were currently undergoing cancer treatment. The minimum study duration was 1 year (≥52 weeks) reflecting metabolic studies suggesting 6 months is the minimum supplementation duration required to equilibrate LCn3 into most body compartments,41 plus a further 6 months to influence cancer development.
Interventions could consist of foods, oral supplements (oil, capsules, or enriched foods) or advice, to increase or decrease omega-3, omega-6 and/or total PUFA intake, or achieve a change of ≥10% of baseline intake, comparing higher versus lower PUFA intake. Studies were excluded if they examined lifestyle or dietary interventions in addition to PUFA unless effects of the PUFA could be separated out.
Primary outcomes included:
New diagnosis of breast cancer
Breast cancer mortality
New diagnosis of any cancer
Any cancer mortality
Secondary outcomes included prostate cancer diagnosis and mortality (added post-hoc to complement prostate-specific antigen (PSA) data), markers of cancer risk (including breast density and PSA), body weight and measures of adiposity, quality of life, and dropouts.
Methods for identification of studies
We searched Cochrane CENTRAL, Medline and Embase to 27 April 2017, ClinicalTrials.com and WHO International Clinical Trials Registry Platform to September 2016 and reassessed all ongoing trials in December 2018. We checked included trials of relevant systematic reviews, and wrote to authors of included studies for additional trial data, creating a database of trials that randomised participants to increased omega-3, omega-6 or total PUFA compared to lower omega-3, omega-6 or total PUFA.39 From this database, trials with duration of at least 12 months and data collected on any primary outcome were included in this review, even if study objectives were not primarily to assess effects on cancer, or those outcomes were not published.
Study inclusion, data extraction and risk of bias assessment (onto a specially developed form) were conducted independently in duplicate. We assessed Cochrane risk of bias domains42 plus risk from compliance problems and attention bias.39 We considered supplementation trials to be at low summary risk of bias where randomisation, allocation concealment, blinding of participants, personnel and outcome assessors were judged adequate (all other trials were considered at moderate or high risk of bias). Dietary advice trials were at low summary risk of bias where randomisation, allocation concealment and blinding of outcome assessors were assessed adequate.39
Data synthesis
Primary analyses assessed effects of total PUFA, omega-6, LCn3 and ALA using random-effects Mantel–Haenszel meta-analysis (as dietary interventions are heterogeneous by their nature43) in Review Manager 5.3.44 Treatment/control differences in outcomes were combined across studies using risk ratios (RR) or mean differences (MD), the at-risk population included only men for prostate cancer and women for breast cancer. Change from baseline in each arm with standard deviations were used for continuous outcomes where available, otherwise endpoint data were used.43 Pre-specified sensitivity analyses included fixed effects analysis, limiting analysis to studies at low summary risk of bias, and limiting to trials randomising ≥100 participants. At the request of our funders, we added sensitivity analyses limiting to studies at low risk for compliance issues. At the request of referees, we added sensitivity analyses using Peto fixed-effects analysis (creating odds ratios), to ensure that our findings are robust to analysis methods despite the inclusion of trials with rare events. Pre-specified subgroup analyses were conducted for outcomes with ≥8 studies by intervention type, replacement, dose, duration, age, sex and cancer risk (normal cancer risk/ increased risk/ previous cancer).39 We planned to sub-group also by medications used, baseline omega-3, omega-6 or total PUFA intake, pre- or post-menopausal, BMI, ethnicity and omega-3/omega-6 ratio, however, this information was not available in most trials, so subgrouping was not attempted. Heterogeneity was assessed using I2 and considered important where >50%.45 Small study bias was assessed using funnel plots where at least 10 trials were included in a meta-analysis.46 Data from individual participants were only counted once in any meta-analytical pooling.
Effect sizes were interpreted as agreed with the WHO Nutrition Guidance Expert Advisory Group (NUGAG) Subgroup on Diet and Health who commissioned this research.39 A risk ratio less than 0.92 or greater than 1.08 was considered a potentially relevant clinical effect (RR 0.92–1.08 was considered “little or no effect”), while a mean difference between arms of at least 10% of baseline was required for a relevant clinical effect for markers. Where we found a suggested effect we quantified the effect using number needed to treat for an additional benefit (NNTB) or number needed to treat to cause an additional harm (NNTH).47 Outcome data were interpreted using GRADE assessment, drafted by LH then discussed and agreed with WHO NUGAG.39 Where sensitivity analyses using Mantel–Haenszel or Peto fixed-effects analyses were not consistent with the main random-effects analysis we downgraded (for inconsistency), and where sensitivity analyses including only trials at low summary risk of bias, or only trials with good compliance differed from the main analysis we downgraded (for risk of bias). Where GRADE suggested data of very low-quality we did not interpret effect sizes. Where data were of low-quality, we used the term “may”, moderate-quality evidence warranted “probably” in describing effects. Summary of findings’ (GRADE) tables show effects on all cancers, breast cancer and prostate cancer diagnoses and deaths (marker evidence strengthened or weakened findings for relevant cancers).
Results
We included 47 RCTs (49 comparisons). Thirty-four trials (97,548 participants) assessed effects of LCn3, three (3179 participants) assessed ALA, eight (4976 participants) assessed omega-6 and 9 trials (11,573 participants) assessed total PUFA (Supplementary Fig. 1 and Supplementary Table 1). As several trials assessed multiple PUFA interventions, numbers are not additive. Thirty-eight trials included participants with normal baseline cancer risk, three with cancer risk factors and six trials with previously diagnosed cancer. Most trials provided supplementary capsules, but omega-6 and total PUFA trials often provided dietary advice and/or supplementary foods (enriched margarines or nuts), and one institutional trial provided all food. Mean trial duration was >30 months and trials were conducted in Europe (20 trials), North America (15), Japan (5), Australia/ New Zealand (2), or over several continents (5). Seventeen RCTs were assessed as being at low summary risk of bias (Supplementary Fig. 2, Supplementary Table 1).
Results are discussed briefly here, fuller results are presented in the supplementary materials (Supplementary Figs. 3–5 are funnel plots relating to effects of LCn3, Supplementary Figs. 6–9 are forest plots depicting effects of omega-3, omega-6 and total PUFA on cancer-related outcomes, Supplementary Fig. 10 the funnel plot for effects of total PUFA on cancer diagnosis, Supplementary Figs. 11–15 further forest plots, Supplementary Tables 2–6 detail results of all meta-analyses and GRADE table on effects of omega-3, Supplementary Tables 7–9 detail meta-analyses and the GRADE table for omega-6, Supplementary Tables 10–12 are meta-analyses and GRADE table for total PUFA).
Effects of increasing long-chain omega-3
Increasing LCn3 has little or no effect on risk of diagnosis of any cancer (high-quality evidence) and probably has little or no effect on risk of cancer death (moderate-quality evidence). We meta-analysed 27 trials (113,557 participants, 7339 diagnoses, mean duration 32 months, mean dose 1.7 g/d LCn3) assessing effects of LCn3 on cancer diagnosis (RR 1.02, 95% CI 0.98 to 1.07, I2 0%, Fig. 1). This lack of effect was not altered in any sensitivity analysis. There was no suggestion of heterogeneity between trials and the funnel plot did not suggest small study bias (Supplementary Fig. 3). Subgrouping did not suggest effect differences by duration, dose, nutrients replaced, intervention type, age, sex or baseline cancer risk. Eighteen trials (99,336 participants) provided data on 2277 cancer deaths (RR 0.97, 95% CI 0.90 to 1.06, I2 0%, Fig. 2). This lack of effect did not alter in sensitivity analyses or subgrouping and there was no suggestion of small study bias (Supplementary Fig. 4) or heterogeneity.
Increasing LCn3 probably has little or no effect on risk of breast cancer diagnosis (moderate-quality evidence), but effects on breast cancer deaths are unclear as the evidence is of very low-quality (two deaths). We meta-analysed 12 trials (44,295 women, 661 diagnoses, mean duration 48 months, mean dose 1.9 g/d LCn3) assessing effects of LCn3 on breast cancer diagnosis (RR 1.03, 95% CI 0.89–1.20, I2 0%, Fig. 3). This lack of effect did not alter in sensitivity analyses, there was no suggestion of small study bias or heterogeneity. Subgrouping did not suggest differences in effect by duration, dose, replacement, intervention type, age, sex or cancer risk. Breast density data were consistent with little or no effect.
Increasing LCn3 may slightly increase prostate cancer risk (low-quality evidence), but effects on prostate cancer death were unclear (the evidence was very low-quality, five deaths). Seven trials (38,525 men, mean duration 51 months, mean dose 1.2 g/d LCn3) reported on 1021 prostate cancer diagnoses, finding higher risk of prostate cancer in men with increased LCn3 (RR 1.10, 95% CI 0.97–1.24, I2 0%, NNTH 334, Fig. 4). This slight increase in prostate cancer risk was stable to all sensitivity analyses. However, the suggestion of harm was contradicted by PSA data reported in a single large trial (25% reduction, MD −0.13 ng/ml, 95% CI −0.25 to 0.01, 1622 participants). Raised PSA was reported in 12 of 62 participants in another trial (RR 0.47, 95% CI 0.16–1.40), also contradicting the suggested LCn3 harms.
Effects of increasing ALA
Increasing ALA probably has little or no effect on risk of cancer death (moderate-quality evidence) and may slightly increase the risk of prostate cancer diagnosis (low-quality evidence). Data on any cancer diagnoses, breast cancer diagnoses, breast or prostate cancer deaths and breast density were too limited to provide useful information, so effects were unclear.
Two trials (5545 participants, durations 24 and 40 months, doses 2 and 5 g/d ALA) provided data on 123 cancer deaths and meta-analysis suggested little or no effect (RR 1.05, 95% CI 0.74–1.49, I2 0%), which did not alter in sensitivity analyses. The same two trials reported 46 prostate cancer diagnoses in 4010 male participants (RR 1.30, 95% CI 0.72–2.32, NNTH 334, I2 0%). This increased risk was consistent across all sensitivity analyses and supported by a rise in PSA in those taking more ALA in the single large trial (rise of 23% from baseline, MD 0.10 ng/ml, 95% CI −0.03 to 0.23).
Effects of increasing omega-6
Evidence for effects of omega-6 on all cancer outcomes was unclear and of very low-quality (see Supplementary Materials).
Effects of increasing total PUFA
Increasing total PUFA may slightly increase risk of diagnosis of any cancer and cancer death (both low-quality evidence). No trials reported breast cancer deaths or breast density, prostate cancer deaths or PSA and effects on breast and prostate cancer diagnoses were unclear (evidence of very low-quality).
Eight trials (9428 participants, 436 diagnoses, mean duration 39 months, doses ranging from 0.8% of energy to 38% of energy from PUFA) assessed effects of increasing total PUFA on cancer diagnosis (RR 1.19, 95% CI 0.99–1.42, NNTH 125, I2 0%), consistent across sensitivity analysis. While the funnel plot suggested small trials with higher risk ratios may be missing (Supplementary Fig. 10), if such trials were included the risk ratio would increase further. Subgrouping did not suggest important differences due to study duration, PUFA dose, age, sex, baseline cancer risk or replacement but data were limited for assessment of subgroup effects. Four trials (3407 participants, 73 deaths, mean duration 39 months, median dose 7%E from PUFA) reported on cancer deaths (RR 1.10, 95% CI 0.48–2.49, NNTH 500, I2 37%), consistent across all sensitivity analyses.
Secondary outcomes
Effects on body weight and measures of adiposity are reported as primary outcomes in other reviews in this series.33,34,35 No trials reported on quality of life; dropouts are reported in supplementary materials.
Discussion
We included 47 long-term RCTs, randomising 108,194 participants. Increasing LCn3 probably has little or no effect on risk of cancer diagnosis, cancer death or breast cancer diagnosis but may slightly increase prostate cancer risk (NNTH 334). Increasing ALA probably has little or no effect on risk of cancer death but may slightly increase prostate cancer risk (NNTH 334). Effects of omega-6 were unclear. Increasing total PUFA may slightly increase risk of diagnosis of any cancer (NNTH 125) and cancer death (NNTH 500).
Strengths and limitations
Strengths of this systematic review include its large size (47 long-term RCTs including more than 108,000 randomised participants worldwide). Creation of a dataset of RCTs randomising to higher or lower PUFA intakes, regardless of primary and reported outcomes, allowed the inclusion of trials and data that would otherwise have been missed or remained unpublished. This allowed us to include many large and long-term RCTs of PUFAs in populations recruited for health problems other than cancer risk, so allowing us to assess effects of increasing PUFA on diagnosis of cancers in low-risk populations. As meta-analysis of trials with rare events can produce different effect sizes when using different analytical methods we ran sensitivity analyses using Mantel–Haenszel and Peto fixed-effects meta-analyses and compared the results with the main random-effects Mantel–Haenszel analysis.48,49,50,51 This ensures that review results are robust to analysis methods.
Review limitations include limited available data on effects of increasing ALA, omega-6 and total PUFA. It was notable that doses of total PUFA were highly variable (from 0.8% of energy to almost 38% of energy from total PUFA in trials providing cancer diagnosis data), but the small number of trials made subgrouping by dose uninformative (Supplementary Figure 11). LCn3 results resulted from meta-analyses of mainly supplementary trials, so effects of increasing oily fish consumption are unclear. As poorly concealed allocation is associated with a 40% greater effect size52 and lack of blinding with additional bias53,54 caution is needed in interpreting small effects in weaker trials. As prostate cancer was not a primary outcome in this review, we did not ask trialists for additional prostate cancer data, which means that more information on prostate cancer may be available from existing trials.
What does this study add?
Our review concurs with a systematic review of observational data23 and two including fewer trials (10 and 19 RCTs to our 34) suggesting LCn3 intake is not associated with total cancer risk.15,16 Two previous systematic reviews of trials and observational data suggested there were inadequate data to determine whether LCn3 intake was associated with prostate cancer incidence or progression.26,29 A systematic review of cohort studies assessing relationships between omega-3 and eleven types of cancer found mixed results, including cohorts suggesting both statistically significantly increased and decreased risk for prostate cancer.23 This review is new in suggesting that actively increasing dietary total PUFA may slightly increase the risk of both cancer diagnosis and cancer mortality. A recent systematic review of observational studies suggested no association between total polyunsaturated fat intake and breast cancer risk,22 but as higher PUFA intake is associated with healthier lifestyles small harms may be difficult to spot in observational studies due to confounding. RCT data are insufficient to corroborate or contradict two nested case-control trials suggesting that higher PUFA intake correlates to higher prostate cancer risk.24,25
The small harms resulting from increased LCn3, ALA and total PUFAs need to be balanced against potential gains from the other major cause of morbidity and mortality, cardiovascular disease (Table 1). For example, this review suggests that increasing LCn3 intake may increase the risk of prostate cancer in men, such that 1000 men increasing their LCn3 intake would lead to three additional men being diagnosed with prostate cancer. In a sister review, meta-analysis including 25 RCTs and over 127,000 participants suggests that if 1000 people consume more LCn3 three will avoid death from coronary heart disease. Further analyses suggest that of the 1000 six will avoid a CHD event and one will avoid arrhythmia.55 The balance appears similar for ALA—for every 1000 people increasing their ALA intake two will avoid a CVD event, eleven will avoid arrhythmia but three will be diagnosed with prostate cancer who would not otherwise have been diagnosed (Fig. 5 represents the harms and benefits visually as number of additional diagnoses incurred or avoided per 1000 people increasing their LCn3, ALA or total PUFA intake).55 Increasing total PUFA in 1000 people appears to prevent five people dying from CHD, but two additional people will die from cancer. Sixteen people will be protected from CVD events, nineteen from CHD events, but eight more will be diagnosed with cancer (Fig. 5).34 This suggests that small benefits and small harms of increasing LCn3 intake are likely to be partially balanced out across major sources of morbidity and mortality and indeed increasing LCn3, ALA, omega-6 and total PUFA appear to have little or no effect on all-cause mortality (Table 1).34,35,55
Bars above zero suggest the number of people who would benefit of 1000 people consuming more PUFA (LCn3, ALA or total PUFA), bars below zero suggest the number of people who would be harmed of 1000 people consuming more PUFA. Where the evidence suggests little or no effect zero appears, and where the evidence is of very low quality no data appear. Cancer data are from this review, CVD data from sister Cochrane reviews.34,55
While increasing LCn3 has little or no effect on risk of cancer diagnosis, breast cancer diagnosis or cancer death (moderate and high-quality evidence), trial evidence suggests that increasing omega-3 may slightly increase prostate cancer risk, and increasing total PUFA may slightly increase cancer risk (low-quality evidence), although this could result from very high intakes of PUFA in some trials. Considering both cancer and cardiovascular outcomes, overall health effects of increasing LCn3, ALA, omega-6 and total PUFA appear small.
References
Cancer Research UK. Worldwide Cancer Statistics https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer: Cancer Research UK. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer#heading-One (2019)
Ritchie, H. How many people in the world die from cancer? Our World in Data. Available from: https://ourworldindata.org/how-many-people-in-the-world-die-from-cancer (2019).
Mariotto, A. B., Yabroff, K. R., Shao, Y., Feuer, E. J. & Brown, M. L. Projections of the cost of cancer care in the United States: 2010–2020. J. Natl Cancer Inst. 103,117–128 (2011)
World Health Organization (WHO). Global Status Report on Noncommunicable Diseases 2014. Geneva, Switzerland. http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf?ua=1: (WHO, 2015).
Barnes, P. M., Bloom, B. & Nahin, R. L. Complementary and alternative medicine use among adults and children: United States, 2007. (US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, Hyattsville, MD, 2008).
Gleissman, H., Johnsen, J. I. & Kogner, P. Omega-3 fatty acids in cancer, the protectors of good and the killers of evil? Exp. Cell Res. 316, 1365–1373 (2010).
Diggle, C. P. In vitro studies on the relationship between polyunsaturated fatty acids and cancer: tumour or tissue specific effects? Prog. Lipid Res. 41, 240–253 (2002).
Hooper, L., Abdelhamid, A., Bunn, D., Brown, T., Summerbell, C. D. & Skeaff, D. M. Effects of total fat intake on body weight. Cochrane Database Syst Rev. 8, CD011834. https://doi.org/10.1002/14651858.CD011834, (2015).
Hajeb, P., Sloth, J. J., Shakibazadeh, S., Mahyudin, N. & Afsah-Hejri, L. Toxic elements in food: Occurrence, binding, and reduction approaches. Compr. Rev. Food Sci. Food Saf. 13, 457–472 (2014).
Malisch, R. & Kotz, A. Dioxins and PCBs in feed and food—Review from European perspective. Sci. Total Environ. 491-492, 2–10 (2014).
Matés, J. M., Segura, J. A., Alonso, F. J. & Márquez, J. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic. Biol. Med. 49, 1328–1341 (2010).
Mozaffarian, D. & Rimm, E. B. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 296, 1885–1899 (2006).
de Lorgeril, M., Salen, P., Martin, J., Monjaud, I., Boucher, P. & Mamelle, N. Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch. Intern. Med. 158, 1181–1187 (1998).
MacLean, C. H., Newberry, S. J., Mojica, W. A., Khanna, P., Issa, A. M., Suttorp, M. J. et al. Effects of omega-3 fatty acids on cancer risk. a systematic review. JAMA 295, 403–415 (2006).
Hooper, L., Thompson, R. L., Harrison, R. A., Summerbell, C. D., Ness, A. R., Moore, H. J. et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: Systematic review. Br. Med. J. 332, 752–755 (2006).
Zhang, Y.-F., Gao, H.-F., Hou, A.-J. & Zhou, Y.-H. Effect of omega-3 fatty acid supplementation on cancer incidence, non-vascular death, and total mortality: a meta-analysis of randomized controlled trials. BMC Public Health 14, 1 (2014).
Trichopoulou, A., Lagiou, P., Kuper, H. & Trichopoulos, D. Cancer and Mediterranean dietary traditions.Cancer Epidemiol. Biomarkers Prev. 9, 869–873 (2000).
Toledo, E., Salas-Salvadó, J., Donat-Vargas, C., Buil-Cosiales, P., Estruch, R., Ros, E. et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: a randomized clinical trial. JAMA Intern. Med. 175, 1752–1760 (2015).
Brasky, T. M., Lampe, J. W., Potter, J. D., Patterson, R. E. & White, E. Specialty supplements and breast cancer risk in the vitamins and lifestyle (VITAL) cohort. Cancer Epidemiol. Biomark. amp; Prev. 19, 1696–1708 (2010).
Fabian, C. J., Kimler, B. F. & Hursting, S. D. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 17, 62 (2015).
Engeset, D., Alsaker, E., Lund, E., Welch, A., Khaw, K.-T., Clavel-Chapelon, F. et al. Fish consumption and breast cancer risk. The European Prospective Investigation into Cancer and Nutrition (EPIC). Int. J. Cancer 119, 175–182 (2006).
Norat, T., Chan, D., Vingeliene, S., Aune, D., Polemiti, E., Vieira, A. R. et al. The Associations between Food, Nutrition and Physical Activity and the Risk of Breast Cancer: World Cancer Research Fund International Systematic Literature Review, 13 Jan 2–17 (Imperial College London,London, 2017)
MacLean, C. H., Newberry, S. J., Mojica, W. A. et al. Effects of omega-3 fatty acids on cancer risk: a systematic review. JAMA 295, 403–415 (2006).
Brasky, T. M., Till, C., White, E., Neuhouser, M. L., Song, X., Goodman, P. et al. Serum phospholipid fatty acids and prostate cancer risk: results from the prostate cancer prevention trial. Am. J. Epidemiol. 173, 1429–1439 (2011).
Brasky, T. M., Darke, A. K., Song, X., Tangen, C. M., Goodman, P. J., Thompson, I. M. et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J. Natl Cancer Inst. 105, 1132–1141 (2013).
Aucoin, M., Cooley, K., Knee, C., Fritz, H., Balneaves, L. G., Breau, R. et al. Fish-derived omega-3 fatty acids and prostate cancer: a systematic review. Integr. Cancer Therapies 16, 32–62 (2016).
Department of Health. Dietary Reference Values: a guide. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/743790/Dietary_Reference_Values_-_A_Guide__1991_.pdf (HMSO, London, 1991).
Forouhi, N. G., Krauss, R. M., Taubes, G. & Willett, W. Dietary fat and cardiometabolic health: evidence, controversies, and consensus for guidance. BMJ. 361, k2139 (2018).
Hackshaw-McGeagh, L. E., Perry, R. E., Leach, V. A., Qandil, S., Jeffreys, M., Martin, R. M. et al. A systematic review of dietary, nutritional, and physical activity interventions for the prevention of prostate cancer progression and mortality. Cancer Causes Control 26, 1521–1550 (2015).
Zheng, J.-S., Hu, X.-J., Zhao, Y.-M., Yang, J. & Li, D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. BMJ 346, f3706 (2013).
Flower, G., Fritz, H., Balneaves, L. G., Verma, S., Skidmore, B., Fernandes, R. et al. Flax and breast cancer: a systematic review. Integr. Cancer Therapies 13, 181–192 (2014).
Ghazanfarpour, M., Sadeghi, R., Roudsari, R. L., Khadivzadeh, T., Khorsand, I., Afiat, M. et al. Effects of flaxseed and Hypericum perforatum on hot flash, vaginal atrophy and estrogen-dependent cancers in menopausal women: a systematic review and meta-analysis. Avicenna J. Phytomedicine 6, 273–283 (2016).
Abdelhamid, A. S., Brown, T. J., Brainard, J. S., Biswas, P., Thorpe, G. C., Moore, H. J. et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev.11:CD003177. https://doi.org/10.1002/14651858.CD003177.pub4 (2018)
Abdelhamid, A. S., Martin, N., Bridges, C., Brainard, J. S., Wang, X., Brown, T. J. et al. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 11:CD012345 https://doi.org/10.1002/14651858.CD012345.pub3 (2018)
Hooper, L., Al-Khudairy, L., Abdelhamid, A. S., Rees, K., Brainard, J. S., Brown, T. J. et al. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 11:CD011094 https://doi.org/10.1002/14651858.CD011094.pub4 (2018)
Brown, T. J., Brainard, J., Song, F., Wang, X., Abdelhamid, A. & Hooper, L. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ 366, l4697 (2019).
Deane, K. H. O., Jimoh, O. F., Biswas, P., O’Brien, A., Hanson, S., Abdelhamid, A. S. et al. Omega-3 and polyunsaturated fat for prevention of depression and anxiety symptoms: systematic review and meta-analysis of randomised trials. Br. J. Psychiatry https://doi.org/10.1192/bjp.2019.234 (2019).
Abdelhamid, A., Hooper, L., Sivakaran, R., Hayhoe, R. P. G. & Welch, A., The PUFAH Group. The relationship between omega-3, omega-6 and total polyunsaturated fat and musculoskeletal health and functional status in adults: a systematic review and meta-analysis of RCTs. Calcif. Tissue Int. 105, 353–372 (2019).
Hooper, L., Abdelhamid, A., Brainard, J., Deane, K. H. O. & Song, F. Creation of a database to assess effects of omega-3, omega-6 and total polyunsaturated fats on health: database and methodology for a set of reviews. BMJ Open. 9, e029554 (2019).
Hanson, S., Thorpe, G., Winstanley, L., Abdelhamid, A. & Hooper, L. Effects of supplementary dietary polyunsaturated fat on cancer incidence. Prospero. CRD42017056109 (2017).
Browning, L. M., Walker, C. G., Mander, A. P., West, A. L., Madden, J., Gambell, J. M. et al. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 96, 748–758 (2012).
Higgins, J. P. T., Altman, D. G. & Sterne, J. A. C. Cochrane Statistical Methods Group, Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. (eds Higgins J. P. T., Green S.). Cochrane handbook for systematic reviews of interventions Version 510 [updated March 2011]. Available from www.handbook.cochrane.org: (The Cochrane Collaboration, 2011).
McKenzie, J. E., Herbison, G. P. & Deeks, J. J. Impact of analysing continuous outcomes using final values, change scores and analysis of covariance on the performance of meta-analytic methods: a simulation study. Res. Synth. Methods 7, 371–386 (2016).
Review Manager 5 (RevMan 5). Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration (2014).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple graphical test. Br. Med J. 315, 629–634 (1997).
Altman, D. G. Confidence intervals for the number needed to treat. BMJ 317, 1309–1312 (1998)
Analysing data and undertaking meta-analyses. Chapter 10. Cochrane Handbook for Systematic Reviews of Interventions version 60 (eds Deeks, J. J., Higgins, J. P. T. & Altman, D. G.). (updated July 2019). Available from www.training.cochrane.org/handbook (Cochrane, 2019).
Efthimiou, O. Practical guide to the meta-analysis of rare events. Evid. Based Ment. Health 21, 72–76 (2018).
Sweeting, M. J., Sutton, A. J. & Lambert, P. C. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat. Med. 23, 1351–1375, https://doi.org/10.1002/sim.1761 (2004).
Sweeting, M. J., Sutton, A. J. & Lambert, P. C. Correction to sweeting 2004. Stat. Med. 25, 2700 (2006).
Schulz, K. F., Chalmers, I., Hayes, R. J. & Altman, D. G. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273, 408–412 (1995).
Savovic, J., Jones, H., Altman, D., Harris, R., Jüni, P., Pildal, J. et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technol. Assess. 16, 1–82 (2012).
Wood, L., Egger, M., Gluud, L. L., Schulz, K., Jüni, P., Altman, D. G. et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 336, 601–605 (2008).
Abdelhamid, A. S., Brown, T. J., Brainard, J. S., Biswas, P., Thorpe, G. C., Moore, H. J. et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 3, CD003177. https://doi.org/10.1002/14651858.CD003177.pub5 (2020)
Acknowledgements
This review is one of a set of reviews conducted by the PUFAH group. The review authors thank all of the authors of primary studies who kindly replied to our queries and where possible provided us with the best set of data available, including: D. Kromhout, Wageningen University (AlphaOmega—ALA; AlphaOmega—EPA + DHA); Emily Chew, NIH (AREDS2 2014); H.S. Black, Veterans Affairs Medical Center, Houston, USA (Black 1994); M.L. Burr, University of Wales and A Ness, University of Bristol (DART fat Burr 1989; DART fish Burr 1989; DART2 Burr 2003); S. Tokudome, National Institute of Health and Nutrition, Japan (DIPP Tukudome 2015); G. Einvik, Akershus University Hospital and H. Arnesen, Oslo University Hospital (DO IT Einvik 2010); A. Sanyal, Virginia Commonwealth University, USA (EPE-A Sanyal 2014); B.G. Feagan, University of Western Ontario, Canada (EPIC-1 2008; EPIC-2 2008); C. Hill, Queen Elizabeth Hospital, Australia (FOSTAR 2016); P.A. Metcalf, University of Aukland, New Zealand (Ley 2004); M. McIllmurray, retired (McIllmurray 1987); J.A. Heady, MRC Statistician (retired) (MRC 1968); D. Nilsen, University of Bergen, Norway (OFAMI Nilsen 2001); S. Schneider, Institut für Herzinfarktforschung, Germany (OMEGA 2009); B.K. Puri, Imperial College London (Puri 2005); C. Roncaglioni and I. Marzona, IRCCS-Istituto di Ricerche Farmacologiche Mario Negri, Italy (Risk & Prevention 2013); P. Rossing, Steno Diabetes Center, Denmark (Rossing 1996); A. Manni, Penn State College of Medicine, USA (Sandhu 2016); C. von Schacky, Ludwig Maximilians University, Munich (SCIMO von Schacky 1999); M.S. Simon, Wayne State University, USA (Simon 1997); P. Galan, Université Paris (SU.FOL.OM3 Galan 2010); K. Tuttle, Sacred Heart Medical Center, Spokane (THIS DIET 2008); J. Sabaté, Loma Linda University, USA (WAHA 2016).
The PUFAH group
Asmaa Abdelhamid2, Sarah Ajabnoor2, Faye Alabdulghafoor2, Lena Alkhudairy3, Priti Biswas4, Julii Brainard2, Charlene Bridges5, Tracey J Brown2, Katherine Deane4, Daisy Donaldson2, Sarah Hanson4, Lee Hooper2, Oluseyi Florence Jimoh2, Nicole Martin5, Alex O’Brien2, Karen Rees3, Lena Alkhudairy3, Fujian Song2, Gabrielle Thorpe4, Xia Wang2, Lauren Winstanley2
Author information
Authors and Affiliations
Consortia
Contributions
S.H., A.S.A. and L.H. designed the study in consultation with the funder. L.H. and A.S.A. built the search. All authors and other members of the PUFAH consortium screened studies and trial registers for eligibility, extracted data and assessed risk of bias. S.H., G.T., L.W., L.H. and A.S.A. input data into Review Manager software, performed the statistical analysis and interpreted the results. S.H. and L.H. wrote the first draft of the paper. L.H. undertook the GRADE analysis and assembled revisions. All authors have read and approved the final version. All authors had full access to all data (primary publications, trials registry entries, trial author communications, data extractions and assessments of risk of bias, and analyses) and take responsibility for the integrity and accuracy of the data. L.H. is the guarantor.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethical approval was required as this was a systematic review and did not use primary data.
Consent to publish
Not applicable.
Data availability
The dataset for this review was part of our published dataset, and so is publicly available, see ref. 39
Competing interests
S.H., G.T., A.S.A. and L.H. had financial support via the University of East Anglia from the World Health Organization for the submitted work, and L.H. and A.A. were also funded to attend WHO meetings and present review results; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Funding information
The set of reviews was commissioned and funded by the World Health Organization (WHO) Nutrition Guidance Expert Advisory Group (NUGAG) Subgroup on Diet and Health, in order to inform their guidance on polyunsaturated fatty acid intake (project number HQNHD1612458). They requested specific inclusion criteria (including duration of trials and outcomes), some sensitivity analyses and subgroups. The results of the reviews, including GRADE assessments, were discussed and reviewed by the WHO NUGAG Subgroup on Diet and Health as part of WHO’s guideline development process. WHO was not otherwise involved in writing this report.
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of the PUFAH group are listed above the Acknowledgements.
Supplementary information
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hanson, S., Thorpe, G., Winstanley, L. et al. Omega-3, omega-6 and total dietary polyunsaturated fat on cancer incidence: systematic review and meta-analysis of randomised trials. Br J Cancer 122, 1260–1270 (2020). https://doi.org/10.1038/s41416-020-0761-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-0761-6
This article is cited by
-
The lipidomic profile of the tumoral periprostatic adipose tissue reveals alterations in tumor cell’s metabolic crosstalk
BMC Medicine (2022)
-
Recent insights into dietary ω-6 fatty acid health implications using a systematic review
Food Science and Biotechnology (2022)
-
Altered Plasma Fatty Acids Associate with Gut Microbial Composition in Common Variable Immunodeficiency
Journal of Clinical Immunology (2022)