Abstract
Small cell lung cancer (SCLC) is characterised by high relapse rates. Tumour-initiating cells (TICs) are responsible for drug resistance and recurrence of cancer. Rovalpituzumab tesirine (Rova-T), a potent humanised antibody–drug conjugate, selectively targets delta-like protein 3, which is highly expressed in SCLC TICs. The experimental drug CBL0137 (CBL) inhibits the histone chaperone FACT (facilitates chromatin transcription), which is required for the expression of transcription factors that are essential for TIC maintenance. Rova-T and CBL each target SCLC TICs as single agents. However, acquired or intrinsic resistance to single agents is a major problem in cancer. Therefore, we investigated the potential effect of combining Rova-T and CBL in SCLC to eradicate TICs more effectively. Our preclinical studies report a novel and highly translatable therapeutic strategy of dual targeting TICs using Rova-T in combination with CBL to potentially increase survival of SCLC patients.
Similar content being viewed by others
Background
Small cell lung cancer (SCLC) has very high mortality because of its high relapse rate after standard-of-care therapies, coupled with a lack of effective second-line therapies. Tumour-initiating cells (TICs) within most solid tumours, including SCLC,1 are important contributors to disease recurrence, metastasis and therapeutic resistance.2,3 TICs can be identified by a high expression of specific marker proteins, such as CD133, compared with the bulk tumour cell population.4
Rovalpituzumab tesirine (Rova-T) is an antibody–drug conjugate (ADC) that comprises a humanised anti-delta-like protein 3 (DLL3) monoclonal antibody attached to a DNA-damaging pyrrolobenzodiazepine toxin.5 Rova-T is considered to be the first biomarker-directed treatment for SCLC and is particularly effective against TICs.5
CBL0137 (CBL) targets FACT (facilitates chromatin transcription), a histone chaperone that is expressed at high levels in tumours and is required for the expression of transcription factors that are essential for TIC maintenance.6,7 Recently, we reported that CBL as a single agent preferentially targets TICs in SCLC and has potent anti-cancer activity against SCLCs when combined with cisplatin.8
Thus, the TIC-targeting mechanisms of CBL and Rova-T are entirely different, targeting two different proteins, FACT and DLL3, respectively, that are highly expressed in SCLC TICs. Here, we investigated the therapeutic efficacy of these drugs in combination using both in vitro and patient-derived xenograft (PDX) models of SCLC. PDXs are well recognised as predictors of human cancer biology and patient response to treatment. The objective of these preclinical studies was to report a novel and highly translatable therapeutic strategy of dual targeting TICs that can potentially increase survival of SCLC patients.
Methods
The CD133high (TIC) and CD133low (non-TIC) cells were generated from NCI-H82 (H82) and NCI-H526 (H526) as described previously.8 The PDX tumour (JHU-LX102), derived from a chemotherapy-naive SCLC patient, was a generous gift from Dr. Charles M. Rudin (Memorial Sloan Kettering Cancer Center, New York). NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice, commonly known as the NOD scid IL-2 receptor gamma knockout (NSG) mice, were inoculated in both flanks with 50,000 tumour cells in each and randomised into cohorts of four animals per arm with mean starting tumour volumes of 100 mm3. Mice were treated with vehicle control for CBL + IgG control, Rova-T alone, CBL alone or the combination of Rova-T with CBL.5,8 Tumour volumes were measured three times a week until vehicle-treated mice reached ~1200 mm3, at which time all mice were euthanised using a gradient controlled CO2 inhalation, followed by cervical dislocation, and the tumours removed. In a second experiment, tumour-bearing mice were treated with the vehicle controls and the drugs as above, but now each group was treated until tumours reached the maximum size of ~1200 mm3. For in vivo limiting dilution assays, cell suspensions from residual tumours harvested from the tumorigenicity studies were inoculated subcutaneously in limiting dilutions (103–105) into naive NSG mice.9 The TIC frequency (TIF) was calculated using the ELDA software (http://bioinf.wehi.edu.au/software/elda/).10
Results
Combination of Rova-T and CBL increases anti-tumour efficacy in vitro and in vivo by decreasing tumour-initiating frequency
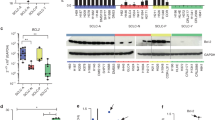
Rova-T kills SCLC TICs by targeting DLL3.5 We observed a higher expression of DLL3 in TICs of H82 and H526 compared to non-TICs (Supplementary Fig. 1A–C), and treatment with CBL had no effect on DLL3 expression in TICs (Supplementary Fig. 1D), suggesting that CBL does not interfere with Rova-T efficacy by decreasing DLL3 levels. The combination of Rova-T and CBL decreased cell survival in H82 and H526 TICs much better than either drug alone (Fig. 1a, b). Importantly, the drug combination had no additional effect on the sensitivity of non-TICs compared to the single drugs alone (Fig. 1c, d), emphasising the preferential targeting of TICs by these drugs.
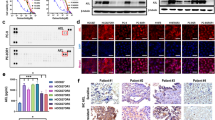
a H82 TICs, b H526 TICs, c H82 non-TICS and d H526 non-TICs were seeded at 3000 cells per well in black-walled 96-well plates. The next day cells were treated with IgG control or Rova-T at different concentrations, or CBL, or with Rova-T + CBL. The cell viability after 72 h of treatment was determined using the CyQUANT Fluorescent Assay8 and normalised to controls. The experiments were repeated thrice, and each measurement was performed in triplicate. Results are represented by means ± SD. Data were analysed using Student’s t test. P values of <0.05 are considered statistically significant. *P < 0.05, and ***P < 0.001. e, f Tumour volumes in mice after treatment with the combination of Rova-T and CBL. Four PDX mice with tumours in both the flanks (N = 8) were treated with vehicle controls for CBL + IgG control, Rova-T alone (1.8 mg/kg, i.p.), CBL alone (60 mg/kg, i.v., once per week) or combinations of Rova-T with CBL. Mice were treated with vehicle controls or CBL + IgG control, Rova-T alone, CBL alone or combinations of Rova-T with CBL. e Tumour volumes were measured in all groups until they reached ~1200 mm3 in the vehicle-treated mice. f The treatment at the same doses as above was continued until the tumour volumes reached ~1200 mm3 in each group. Tumour volumes (v) were calculated using the volume for a prolate spheroid: v = 4/3 × π × a2 × b, where a is the minor radius and b the major radius. Differences between groups were analysed by the Student’s t test. The results are represented as means ± SE. * indicates P < 0.05 versus the single drug treatment groups. g Survival of mice shown in f (P < 0.05 for the combination vs single drugs alone). h SOX2 protein level was determined by Western analysis in the residual tumours derived from mice after treatment with vehicle for CBL + IgG control, or with CBL, Rova-T or Rova-T + CBL. β-Actin was used as a loading control. i In vivo limiting dilution assay showing that combining Rova-T and CBL reduced the tumour-initiating frequency (TIF). Mice were scored positive for tumour growth when the tumour size exceeded 200 mm3 at 6 months after tumour cell inoculation. The TIF was calculated from N = 8 mice per group for each dilution of cells.
We compared the anti-tumour efficacy of Rova-T combined with CBL against single agents in a SCLC PDX model. There was no significant reduction in tumour growth in the mice treated with Rova-T plus CBL until day 50 compared to the groups treated with the individual drugs. However, tumour sizes started decreasing significantly (P < 0.05) after day 55 in the combination group (Fig. 1e). Since the vehicle-treated group had already reached the maximum allowed size by that day, we sacrificed the mice in all groups at that time. In a second experiment, tumour-bearing mice were treated with the vehicle controls or drugs at the same doses as above, but in this experiment, the mice in each group were treated until their tumours reached the maximum size of ~1200 mm3. Treatment was started on day 31 after inoculation. Rova-T in combination with CBL substantially inhibited tumour growth, compared to Rova-T alone (P < 0.05), CBL alone (P < 0.05) or vehicle control (P < 0.05) (Fig. 1f). We observed that mice treated with combined Rova-T and CBL survived for 133 days, whereas vehicle-treated mice survived for 51 days, and mice treated with either CBL or Rova-T survived for 74–77 days (Fig. 1g). We could also show that the well-established cancer stem cell marker SOX2 level was decreased in the residual tumours from mice treated with CBL and Rova-T, compared to controls, with the drug combination being most effective (Fig. 1h). Rova-T treatment decreased DLL3 expression in residual tumours (Supplementary Fig. 2), indicating that Rova-T kills DLL3-expressing TICs. These results indicate that Rova-T in combination with CBL decreased the growth of SCLC PDX tumours and also increased survival by killing TICs, revealing a novel potent combination therapy for this cancer. We did not observe any toxicity in animals during treatment.
The limiting dilution assay is a rigorous test to quantitate cellular tumour-initiating capacity within a heterogeneous cancer cell population. Both Rova-T5 and CBL7,8 as single agents reduce tumour-initiating capacity in vivo4 or in vitro.7,8 To determine whether Rova-T combined with CBL can reduce tumour recurrence by targeting TICs, we performed in vivo limiting dilution assays on residual tumours. Tumours derived from control mice were shown to have a TIF of 1:1424, which was reduced to 1:1961 and 1:2598 in CBL or Rova-T-treated mice, respectively. A substantial reduction in the TIF to 1:11,871 was observed in tumours derived from mice treated with the combination of Rova-T and CBL (Fig. 1i).
Discussion
SCLCs contain a much higher percentage of TICs than non-SCLCs, 65–75% compared to 15–20%,11 and may therefore represent an ideal cancer in which to target TICs, which are relatively insensitive to chemotherapy and seed the growth of newly resistant tumours. While Rova-T and CBL have each been shown to target SCLC TICs as single agents,5,8 it is unlikely that any drug will be curative as a single agent. Our findings show that the combination of two different TIC inhibitors, targeting two different proteins, DLL3 and FACT, is much more toxic to SCLC TICs than either drug alone, and this therapeutic strategy is effective in vivo.
Tumour TICs can self-renew, differentiate and give rise to a new tumour. We reveal that the combination of Rova-T and CBL decreases SOX2 and attenuates the in vivo self-renewal capability of SCLC tumours by eradicating TICs, and thereby may also help to counteract relapse.
CBL is currently in the final stages of multicentre phase I clinical trials in advanced or metastatic solid tumours and lymphomas (NCT01905228), and it has not yet exhibited dose-limiting toxicity. Therefore, using CBL in combination with Rova-T may add therapeutic value to disappointing recent results with Rova-T alone in SCLC,12,13 and could represent a novel drug combination that can prevent tumour recurrence and yield a more durable response in this cancer.
References
Codony-Servat, J., Verlicchi, A. & Rosell, R. Cancer stem cells in small cell lung cancer. Transl. Lung Cancer Res. 5, 16–25 (2016).
Wang, J., Li, Z. H., White, J. & Zhang, L. B. Lung cancer stem cells and implications for future therapeutics. Cell Biochem. Biophys. 69, 389–398 (2014).
Eramo, A., Haas, T. L. & De Maria, R. Lung cancer stem cells: tools and targets to fight lung cancer. Oncogene 29, 4625–4635 (2010).
MacDonagh, L., Gray, S. G., Breen, E., Cuffe, S., Finn, S. P., O’Byrne, K. J. et al. Lung cancer stem cells: the root of resistance. Cancer Lett. 372, 147–156 (2016).
Saunders, L. R., Bankovich, A. J., Anderson, W. C., Aujay, M. A., Bheddah, S., Black, K. et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci. Transl. Med. 7, 302ra136 (2015).
Gasparian, A. V., Burkhart, C. A., Purmal, A. A., Brodsky, L., Pal, M., Saranadasa, M. et al. Curaxins: anticancer compounds that simultaneously suppress NF-kappaB and activate p53 by targeting FACT. Sci. Transl. Med. 3, 95ra74 (2011).
Dermawan, J. K., Hitomi, M., Silver, D. J., Wu, Q., Sandlesh, P., Sloan, A. E. et al. Pharmacological targeting of the histone chaperone complex FACT preferentially eliminates glioblastoma stem cells and prolongs survival in preclinical models. Cancer Res. 76, 2432–2442 (2016).
De, S., Lindner, D. J., Coleman, C., Wildey, G., Dowlati, A., Stark, G. R. et al. The FACT inhibitor CBL0137 synergizes with cisplatin in small cell lung cancer by increasing NOTCH1 expression and targeting tumor-initiating cells. Cancer Res. 78, 2396–2406 (2018).
Kolev, V. N., Wright, Q. G., Vidal, C. M., Ring, J. E., Shapiro, I. M., Ricono, Jill, D. et al. PI3K/mTOR dual inhibitor VS-5584 preferentially targets cancer stem cells. Cancer Res. 75, 446–455 (2015).
Hu, Y. & Smyth, G. K. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods 347, 70–78 (2009).
Sullivan, J. P., Spinola, M., Dodge, M., Raso, M. G., Behrens, C., Gao, B. et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 70, 9937–9948 (2010).
Van Den Borg, R., Leonett, A., Tiseo, M., Giovannetti, E. & Peters, G. J. Novel targeted strategies to overcome resistance in small-cell lung cancer: focus on PARP inhibitors and rovalpituzumab tesirine. Expert Rev. Anticancer Ther. 19, 461–471 (2019).
Morgensztern, D., Besse, B., Greillier, L., Davila, R. S., Ready, N., Christine, L. et al. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: results from the Phase II TRINITY Study. Clin. Cancer Res. 25, 6958–6966 (2019).
Acknowledgements
We are extremely thankful to Drs. Andrei Gudkov and Andrei A. Purmal of Incuron Inc. for providing CBL0137. We would like to thank AbbVie Inc. for providing Rova-T, and Dr. Shawn Jeffries, the clinical director of AbbVie, for his support and scientific input. We appreciate the technical support of the Cleveland Clinic Flow Cytometry Core.
Author information
Authors and Affiliations
Contributions
D.J.L. and Y.P. performed the in vivo experiments and S.D. the in vitro experiments. D.J.L., G.W., A.D, G.R.S. and S.D. were involved in designing the study, obtaining research materials and editing the paper. S.D. supervised the study and wrote the initial manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experiments were approved by the Cleveland Clinic Foundation Institutional Animal Care and Use Committee and conducted in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals (Protocol Number: 2017-1863). The cell lines used in this study were obtained from ATCC.
Consent to publish
Not applicable.
Data availability
All data generated or analysed during this study are included in this article and its supplementary information files.
Competing interests
The authors declare no competing interests.
Funding information
This research was mainly supported by a Concept Grant (LC170491) from the US Department of Defense (DoD) to S.D. Studies were supported, in part, by the Case Comprehensive Cancer Center Athymic Animal and Xenograft Core and NCI core grant P30 CA043703-23.
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lindner, D.J., Wildey, G., Parker, Y. et al. CBL0137 increases the targeting efficacy of Rovalpituzumab tesirine against tumour-initiating cells in small cell lung cancer. Br J Cancer 124, 893–895 (2021). https://doi.org/10.1038/s41416-020-01192-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-01192-x
This article is cited by
-
The FACT-targeted drug CBL0137 enhances the effects of rituximab to inhibit B-cell non-Hodgkin’s lymphoma tumor growth by promoting apoptosis and autophagy
Cell Communication and Signaling (2023)
-
Kidney cancer biomarkers and targets for therapeutics: survivin (BIRC5), XIAP, MCL-1, HIF1α, HIF2α, NRF2, MDM2, MDM4, p53, KRAS and AKT in renal cell carcinoma
Journal of Experimental & Clinical Cancer Research (2021)