Abstract
Background
The impact of various breast-cancer treatments on patients with a BRCA2 mutation has not been studied. We sought to estimate the impact of bilateral oophorectomy and other treatments on breast cancer-specific survival among patients with a germline BRCA2 mutation.
Methods
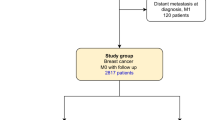
We identified 664 women with stage I–III breast cancer and a BRCA2 mutation by combining five different datasets (retrospective and prospective). Subjects were followed for 7.2 years from diagnosis to death from breast cancer. Tumour characteristics and cancer treatments were patient-reported and derived from medical records. Predictors of survival were determined using Cox proportional hazard models, adjusted for other treatments and for prognostic features.
Results
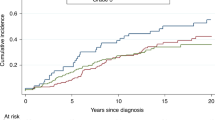
The 10-year breast-cancer survival for ER-positive patients was 78.9% and for ER-negative patients was 82.3% (adjusted HR = 1.23 (95% CI, 0.62–2.45, p = 0.55)). The 10-year breast-cancer survival for women who had a bilateral oophorectomy was 89.1% and for women who did not have an oophorectomy was 59.0% (adjusted HR = 0.45; 95% CI, 0.28–0.72, p = 0.001). The adjusted hazard ratio for chemotherapy was 0.83 (95% CI, 0.65–1.53: p = 0.56).
Conclusions
For women with breast cancer and a germline BRCA2 mutation, positive ER status does not predict superior survival. Oophorectomy is associated with a reduced risk of death from breast cancer and should be considered in the treatment plan.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
13 January 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41416-022-02130-9
References
Byrski, T., Gronwald, J., Huzarski, T., Grzybowska, E., Budryk, M., Stawicka, M. et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J. Clin. Oncol. 28, 375–379 (2010).
Narod, S. A., Huzarski, T., Gronwald, J., Byrski, T., Marczyk, E., Cybulski, C. et al. Predictors of survival for breast cancer patients with a BRCA1 mutation. Breast Cancer Res. Treat. 168, 513–521 (2018).
Metcalfe, K., Lynch, H. T., Foulkes, W. D., Tung, N., Kim-Sing, C., Olopade, O. I. et al. Effect of oophorectomy on survival after breast cancer in BRCA1 and BRCA2 mutation carriers. JAMA Oncol. 1, 306–313 (2015).
Metcalfe, K., Gershman, S., Ghadirian, P., Lynch, H. T., Snyder, C., Tung, N. et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ 348, g226 (2014).
Jayasekara, H., MacInnis, R. J., Chamberlain, J. A., Dite, G. S., Leoce, N. M., Dowty, J. G. et al. Mortality after breast cancer as a function of time since diagnosis by estrogen receptor status and age at diagnosis. Int. J. Cancer 145, 3207–3217 (2019).
Jonasson, J. G., Stefansson, O. A., Johannsson, O. T., Sigurdsson, H., Agnarsson, B. A., Olafsdottir, G. H. et al. Oestrogen receptor status, treatment and breast cancer prognosis in Icelandic BRCA2 mutation carriers. Br. J. Cancer 115, 776–783 (2016).
Metcalfe, K., Lynch, H. T., Foulkes, W. D., Tung, N., Olopade, O. I., Eisen, A. et al. Oestrogen receptor status and survival in women with BRCA2-associated breast cancer. Br. J. Cancer 120, 398–403 (2019).
Shuster, L. T., Gostout, B. S., Grossardt, B. R. & Rocca, W. A. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 14, 111–116 (2008).
Cybulski, C., Kluźniak, W., Huzarski, T., Wokołorczyk, D., Kashyap, A., Rusak, B. et al. The spectrum of mutations predisposing to familial breast cancer in Poland. Int. J. Cancer 145, 3311–3320 (2019).
Mann, G. J., Thorne, H., Balleine, R. L., Butow, P. N., Clarke, C. L., Edkins, E. et al. Analysis of cancer risk and BRCA1 and BRCA2 mutation prevalence in the kConFab familial breast cancer resource. Breast Cancer Res. 8, R12 (2006).
Phillips, K. A., Butow, P. N., Stewart, A. E., Chang, J. H., Weideman, P. C., Price, M. A. et al. Predictors of participation in clinical and psychosocial follow-up of the kConFab Breast Cancer Family Cohort. Fam. Cancer 4, 105–113 (2005).
Phillips, K. A., Milne, R. L., Buys, S., Friedlander, M. L., Ward, J. H., McCredie, M. et al. Agreement between self-reported breast cancer treatment and medical records in a population-based breast cancer family registry. J. Clin. Oncol. 23, 4679–4686 (2005).
Foulkes, W. D., Metcalfe, K., Sun, P., Hanna, W. M., Lynch, H. T., Ghadirian, P. et al. Estrogen receptor status in BRCA1- and BRCA2-related breast cancer: the influence of age, grade, and histological type. Clin. Cancer Res. 10, 029–2034 (2004).
Olafsdottir, E., Borg, A., Jensen, M. B., Gerdes, A. M., Johansson, A. L. V., Barkardottir, R. B. et al. Breast cancer survival in Nordic BRCA2 mutation carriers—unconventional association with oestrogen receptor status. Br. J. Cancer https://doi.org/10.1038/s41416-020-01056-4 (2020).
Bane, A. L., Beck, J. C., Bleiweiss, I., Buys, S. S., Catalano, E., Daly, M. B. et al. BRCA2 mutation-associated breast cancers exhibit a distinguishing phenotype based on morphology and molecular profiles from tissue microarrays. Am. J. Surg. Pathol. 31, 121–128 (2007).
Goodwin, P. J., Phillips, K. A., West, D. W., Ennis, M., Hopper, J. L., John, E. M. et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J. Clin. Oncol. 30, 19–26 (2012).
Francis, P. A., Pagani, O., Fleming, G. F., Walley, B. A., Colleoni, M., Láng, I. et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N. Engl. J. Med. 379, 122–137 (2018).
Acknowledgements
Our sincere gratitude for the valuable contributions of the women who participated in this study, without whom this research would not be possible. We would like to acknowledge the following individuals for helping with data collection: Sara Lazzarin, Debora Vicini and Federica Sina.
Other members of the Polish Hereditary Breast Cancer Consortium
Błasińska-Morawiec Maria16, Chosia Maria17, Drosik Kazimierz18, Gozdecka-Grodecka Sylwia19, Goźdź Stanisław20, Grzybowska Ewa21, Jeziorski Arkadiusz22, Karczewska Aldona23, Kordek Radzisław22, Synowiec Agnieszka24, Kozak-Klonowska Beata25, Lamperska Katarzyna26, Lange Dariusz27, Mackiewicz Andrzej19, Mituś Jerzy Władysław28, Niepsuj Stanislas29, Oszurek Oleg17, Gugała Karol30, Morawiec Zbigniew16, Mierzwa Tomasz31, Posmyk Michał32, Ryś Janusz33, Szczylik Cezary34, Uciński Michał17, Urbański Krzysztof35, Waśko Bernard36, Wandzel Piotr37
Other members of the kConFab Follow-Up Study Team
Michael Friedlander38, Sue Anne McLachlan39, Stephanie Nesci40, Sandra Picken40, Sarah O’Connor40, Lucy Stanhope40
Other members of Hereditary Breast Cancer Clinical Study Group
Andrea Eisen41, Kevin Sweet42, Raymond Kim43, William Foulkes44, Pal Moller45, Susan Neuhausen46, Carey Cullinane47, Charis Eng48, Peter Ainsworth49, Fergus Couch50, Christian Singer51, Beth Karlan52, Wendy McKinnon53, Marie Wood53
Author information
Authors and Affiliations
Consortia
Contributions
Conception, design and study supervision: S.A.N. Acquisition of data: J.G., J.L., T.H., Z.H., C.F., K.M., L.S., D.Z. and M.E. Analysis of clinical data: R.F., J.L., C.C., D.Z., J.W. and N.T. Statistical analysis and critical review: P.S., R.L.M. and K.M. Interpretation of the results and writing of the original draft: D.G.E., S.A.N. and K.A.P. All authors revised the paper and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the following institutional ethics review boards: Women’s College Hospital Ethics Board, Research Ethics Committee of the Pomeranian Medical University, Central Manchester Research Ethics Committee (10/H1008/24 and 11/H1003/3), Peter MacCallum Cancer Centre Human Research Ethics Committee (97/27) and the Ethical Committee Brianza. All study subjects provided written informed consent. The study was performed in accordance with the Declaration of Helsinki.
Consent to publish
Individual person’s data not included.
Data availability
All data relevant to the study are included in the paper.
Competing interests
Steven A. Narod is a board member of BJC. All other authors declare no conflict of interest.
Funding information
This work was supported by grants to kConFab and the kConFab Follow-Up Study from Cancer Australia (809195, 1100868), the Australian National Breast Cancer Foundation (IF 17), the National Health and Medical Research Council (454508, 288704 and 145684), the National Institute of Health USA (1RO1CA159868), the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania and South Australia and the Cancer Foundation of Western Australia. K.A.P. is an Australian National Breast Cancer Foundation Fellow (PRAC17-004). The work is supported by the Peter Gilgan Center for Research on Women’s Cancers and the Canadian Institute of Health Research (FDN 154275). D.G.E. is supported by the NIHR Manchester Biomedical Research Centre (IS-BRC-1215-20007).
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the Polish Breast Cancer Consortium, kConFab Follow-Up Study Team and Hereditary Breast Cancer Clinical Study Group are listed above Acknowledgements.
The original online version of this article was revised: The original version of this article unfortunately contained a mistake in an author affiliation. The correct affiliations of author Tomasz Huzarski are: Department of Genetics and Pathology, International Hereditary Cancer Center, Pomeranian Medical University, Szczecin, Poland and University of Zielona Góra, Zielona Góra, Poland.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Evans, D.G., Phillips, KA., Milne, R.L. et al. Survival from breast cancer in women with a BRCA2 mutation by treatment. Br J Cancer 124, 1524–1532 (2021). https://doi.org/10.1038/s41416-020-01164-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-01164-1
This article is cited by
-
ER-positive and BRCA2-mutated breast cancer: a literature review
European Journal of Medical Research (2024)
-
Estrogen receptor-positive breast cancer and adverse outcome in BRCA2 mutation carriers and young non-carrier patients
npj Breast Cancer (2023)
-
PREDICT validity for prognosis of breast cancer patients with pathogenic BRCA1/2 variants
npj Breast Cancer (2023)
-
The impact of oophorectomy on survival from breast cancer in patients with CHEK2 mutations
British Journal of Cancer (2022)
-
Adjuvant olaparib — should all patients with breast cancer have genetic testing?
Nature Reviews Clinical Oncology (2021)