Abstract
Background
Regorafenib or trifluridine/tipiracil as third-line treatment have limited efficacy in metastatic colorectal cancer (mCRC).
Methods
This Phase 2 trial evaluated the efficacy and safety of irinotecan plus cetuximab rechallenge as third-line treatment in KRAS wild-type mCRC patients who achieved clinical benefit with first-line cetuximab-containing therapy. The primary endpoint was 3-month progression-free survival (PFS) rate. A sample size was calculated; 30 patients with a 3-month PFS rate of 45% deemed promising and 15% unacceptable. Patients with greater and less than the cut-off value of cetuximab-free intervals (CFIs) were classified into the long and short CFI groups, respectively, in subgroup analyses.
Results
Among 34 eligible patients who received treatment at least once, 3-month PFS rate was 44.1% (95% confidence interval, 27.4–60.8%). The median PFS and overall survival (OS) were 2.4 and 8.2 months, respectively. The response and disease control rates were 2.9 and 55.9%, respectively. PFS and OS were significantly longer in the long- than in the short CFI group.
Conclusions
Irinotecan plus cetuximab rechallenge as third-line treatment for KRAS wild-type mCRC was safe and had promising activity, especially in those with a long CFI, warranting further investigation in a Phase 3 randomised trial.
Clinical trial registration
UMIN000010638
Similar content being viewed by others
Background
5-fluorouracil/leucovorin plus oxaliplatin or irinotecan (FOLFOX or FOLFIRI) in combination with an anti-epidermal growth factor receptor (EGFR) antibody (cetuximab or panitumumab) is a standard first- or second-line therapy in patients with RAS wild-type metastatic colorectal cancer (mCRC).1,2,3 In addition, anti-EGFR antibodies with or without irinotecan are used as standard third-line treatment in patients who have not been previously treated with anti-EGFR antibodies.4,5,6 Patients who are refractory to these drugs can receive regorafenib or trifluridine/tipiracil (FTD/TPI) as a third-line or later treatment option. Although regorafenib and FTD/TPI were shown to achieve a significantly longer overall survival (OS) than placebo in the CORRECT and RECOURSE trials, these drugs have limited efficacy, with a response rate (RR) ranging from 1 to 1.6%.7,8 The development of new drugs and strategies is necessary to improve outcomes in patients receiving third-line treatment.
Santini et al. reported that cetuximab rechallenge had promising efficacy in patients who achieved clinical benefit in response to first-line cetuximab plus chemotherapy. The RR of FOLFIRI (or irinotecan) plus cetuximab rechallenge was 54%, which was much higher than that of regorafenib or FTD/TPI. However, the study by Santini et al. was a single-arm Phase 2 study, and no study had reproduced the efficacy of cetuximab rechallenge at that time.9 Therefore, we conducted a Phase 2 study of irinotecan plus cetuximab rechallenge as third-line treatment in patients with KRAS exon 2 wild-type mCRC (Clinical trial information: UMIN000010638).
Methods
Study design and treatment schedule
This was a Phase 2, multicentre, open-label, non-randomised trial to evaluate the efficacy and safety of irinotecan plus cetuximab rechallenge as third-line treatment in patients with KRAS exon 2 wild-type mCRC. Patients received 150 mg/m2 irinotecan intravenously every 2 weeks. Cetuximab was administered as a 2-h intravenous infusion at a loading dose of 400 mg/m2, followed by weekly 1-h infusions of 250 mg/m2. Irinotecan was allowed to be discontinued after day 15 based on the investigator’s decision. All patients received an H1-histamine antagonist and dexamethasone before cetuximab and 5-hydroxytryptamine-3 receptor antagonist before irinotecan.
The protocol for the present study was reviewed and approved by the Institutional Review Boards of all participating institutions.
Eligibility criteria
Inclusion criteria were (1) histologically confirmed unresectable colorectal adenocarcinoma, (2) KRAS exon 2 wild-type, (3) achievement of complete response, partial response or stable disease for at least 6 months with first-line cetuximab plus doublet chemotherapy, (4) refractoriness or intolerance to two regimens, including oxaliplatin and irinotecan, (5) Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2, (6) measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, (7) adequate bone marrow, hepatic and renal function (white blood cell count, ≥3000/μL and ≤12,000/μL; neutrophil count, ≥1500/μL; haemoglobin, ≥9.0 g/dL; platelet count, ≥100,000/μL; total bilirubin, ≤2.0 mg/dL; aspartate and alanine aminotransferase, ≤100 IU/L or ≤300 IU/L in patients with liver metastases; creatinine, ≤1.5 mg/dL), (8) age > 20 years, (9) life expectancy of at least 3 months and (10) written informed consent. Exclusion criteria were (1) history of malignant tumours within 5 years before the study treatment initiation, (2) symptomatic brain metastases, (3) severe complications (infection, lung and heart disease and liver and renal dysfunction), (4) massive pleural effusion, ascites and pericardial effusion and (5) other serious medically important abnormalities.
Toxicity and dose modifications
Toxicity was assessed according to the National Cancer Institute Common Toxicity Criteria (version 4.0). In the presence of ≥grade 3 skin toxicity, cetuximab dose interruption was required until regression to ≤grade 2. If grade ≥3 skin toxicity occurred two times, the dose of cetuximab was reduced to 200 mg/m2, and if grade ≥3 skin toxicity occurred once more, the dose of cetuximab was reduced to 150 mg/m2. In the presence of ≥grade 2 diarrhoea and ≥grade 3 haematological toxicity, irinotecan dose interruption was required until regression to ≤grade 1 and ≤grade 2, respectively. In the presence of ≥grade 3 haematological toxicity and thrombocytopenia and grade 4 neutropenia, irinotecan dose was sequentially reduced to 120 and 100 mg/m2. Supportive therapy was administered when necessary. The protocol treatment was repeated until disease progression, unacceptable toxicity, death or withdrawal of consent.
Outcome measures and statistical analysis
All patients underwent computed tomography (CT) at chemotherapy initiation and every 8 weeks to evaluate tumour response according to RECIST version 1.1. The primary endpoint was 3-month progression-free survival (PFS). A sample size was calculated; 30 patients with a 3-month PFS rate of 45% deemed promising and 15% unacceptable (one-sided α, 0.05; β, 0.2). The secondary endpoints were RR, disease control rate (DCR), PFS, OS, time to treatment failure (TTF) and safety. RR was defined as the proportion of patients with complete or partial response. DCR was defined as the proportion of patients with complete, partial response or stable disease. Complete, partial response and stable disease were without confirmation of response. PFS was defined from the date of enrolment to the first observation of disease progression or death from any cause. OS was defined from the date of enrolment to death from any cause. PFS and OS were estimated using the Kaplan–Meier method. Deepness of response (DpR) defined as the rate of tumour shrinkage from baseline CT was evaluated as an exploratory analysis.
Post hoc subgroup analysis according to cetuximab-free interval (CFI) was conducted to explore predictive factors for irinotecan plus cetuximab rechallenge. CFI was defined as the interval from the date of the last cetuximab dose in first-line treatment to the date of the first cetuximab dose in the present study. Receiver-operating characteristic (ROC) analysis using a 3-month PFS rate as the second variable was performed to determine an optimal cut-off value of CFI. Patients with greater and less than the optimal cut-off value for CFI were classified into long and short CFI groups, respectively. In subgroup analyses, DCR, rate of DpR > 0%, PFS and OS were compared between the two CFI groups. Several cut-off values of CFI based on a previous report10 were used as it was unclear whether the cut-off value of CFI in the present study was appropriate. All analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC, USA) and R version 3.4.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
The study included 36 patients enrolled between May 2013 and October 2015. One patient could not receive protocol treatment due to rapid disease progression after enrolment in the study. The safety population included patients who received the protocol treatment at least once. Thirty-four of the 35 patients received the protocol treatment. One patient proved to be ineligible after initiation of protocol treatment because no blood evaluation had been performed for this patient before study enrolment. The full analysis set comprised patients meeting the eligibility criteria who received the protocol treatment at least once, and included 34 patients after the exclusion of one ineligible patient.
The study cohort characteristics are summarised in Table 1. Briefly, the median age was 64.5 (range, 41–80) years, 11 patients (32%) were female, 33 patients (97%) had an ECOG PS of 0–1, primary colorectal tumours were not resected in 9 patients (27%) at the time of enrolment in this study, 30 patients (88%) had left-sided tumours (rectum, sigmoid or descending colon), 29 patients (85%) had liver metastases, 8 patients (24%) had peritoneal metastases and 25 patients (77%) had two or more metastatic sites. With first-line cetuximab-containing treatments, 29 patients (85%) had partial response and 5 patients (15%) had stable disease for 6 months or more. In addition, 15 patients (44%) received irinotecan-based regimens as first-line treatment; all 15 patients received oxaliplatin-based regimens as second-line treatment. Among 34 patients, 33 of the 34 patients (97%) received bevacizumab as second-line treatment.
Treatment exposure
The median number of doses was 8 (range, 1–37) for cetuximab and 4 (range, 1–13) for irinotecan, whereas the median relative dose intensity was 80.3% (60.2 mg/m2/week; range, 22.7–80.6 mg/m2/week) for irinotecan and 90.1% (225.3 mg/m2/week; range, 66.0–280.5 mg/m2/week) for cetuximab. Irinotecan and cetuximab dose modifications were required in 8 (23.5%) and 5 (14.7%) patients, respectively, due to adverse events. Irinotecan and cetuximab dose interruptions were required in 18 (52.9%) and 17 (50.0%) patients, respectively. All patients had discontinued irinotecan plus cetuximab at the time of analysis. The median follow-up duration was 13.2 (range, 1.1–29.2) months, and the median TTF was 2.2 months (95% confidence interval [CI], 1.8–3.2 months). The reasons for discontinuation included disease progression in 32 patients (94%), grade 3 skin toxicities that were not resolved within 35 days from the last dose of cetuximab in one patient (3%) and grade 2 anorexia in one patient (3%). There were no treatment-related deaths during the study period.
Efficacy
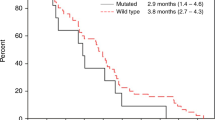
Among 34 patients in the full analysis set, 3-month PFS rate was 44.1% (95% CI, 27.4–60.8%) (Fig. 1). The median PFS and OS were 2.4 months (95% CI, 2.0–3.7 months) and 8.2 months (95% CI, 6.1–11.7 months), respectively (Figs. 1, 2). One patient achieved partial response with an RR of 2.9% (95% CI, 0.07–15.3%), and 18 patients achieved stable disease with a DCR of 55.9% (95% CI, 37.9–72.8%). The median DpR was −6.7% (range, −66.0 to 31.7%), and 9 patients (27%) had a DpR of > 0%.
Subgroup analysis according to CFI
The median CFI was 330 (range, 56–1224) days. The optimal CFI cut-off value of 372 days was determined by ROC analysis (area under the curve = 0.81).
Among 34 patients, 11 and 23 patients were classified into the long and short CFI groups. The DCRs were 82% and 44% for the long and short CFI groups, respectively (p = 0.064). The rate of DpR of >0% was higher in the long- than in the short CFI group (55% vs. 13%, respectively, p = 0.033) (Fig. 3). The CFIs of three patients with a rate of DpR of >0% in the short CFI group were 338, 181 and 176 days, respectively, from the right of patients in Fig. 3. The 3-month PFS rates were 81.8% and 26.1% in the long and short CFI groups, respectively. The median PFS was 4.6 months in the long CFI group and 2.1 months in the short CFI group (hazard ratio, 0.40; 95% CI, 0.18–0.86; p = 0.020) (Fig. 4a). The median OSs were 14.1 and 6.3 months in the long and short CFI groups, respectively (hazard ratio, 0.31; 95% CI, 0.13–0.74, p = 0.008) (Fig. 4b).
The rate of deepness of response >0% is higher in the long- than in the short CFI group (55% vs. 13%, respectively, p = 0.003). The CFIs of three patients with a rate of DpR of >0% in the short CFI group were 338, 181 and 176 days, respectively, from the right of patients. *Patients who discontinued first-line cetuximab-containing treatment due to reasons other than progressive disease. CFI cetuximab-free interval, DpR deepness of response.
a The median progression-free survival is 4.6 months in the long CFI group and 2.1 months in the short CFI group (hazard ratio, 0.40; 95% CI, 0.18–0.86; p = 0.020). b The median overall survival is 14.6 months in the long CFI group and 6.3 months in the short CFI group (hazard ratio, 0.31; 95% CI, 0.13–0.74; p = 0.008). CFI cetuximab-free interval, CI confidence interval.
Using several cut-off values of CFI, including 4.4, 6 and 8.8 months, the PFS and OS were longer in the long- than in the short CFI group (Supplementary Figs. 1, 2 and Supplementary Table 1).
Adverse events
Among 35 patients in the safety population, common grade 3–4 adverse events included neutropenia (n = 10, 29%), anaemia (n = 2, 6%), elevated aspartate aminotransferase (n = 2, 6%), anorexia (n = 2, 6%) and fatigue (n = 2, 6%). There were no grade 3–4 skin toxicities. Common grade 1–2 skin toxicities were skin rash (n = 28, 80%), dry skin (n = 20, 57%) and paronychia (n = 16, 46%) (Table 2).
Discussion
In the present Phase 2 study, we demonstrated that irinotecan plus cetuximab rechallenge as third-line treatment was safe and had promising activity in patients with KRAS wild-type mCRC who exhibited clinical response to cetuximab plus cytotoxic agents as first-line chemotherapy, warranting further investigation in a Phase 3 randomised trial. Specifically, patients with long CFIs might be good candidates for cetuximab rechallenge compared to those with short CFIs. To our knowledge, the present study is the first to show long CFI as a predictive marker for the efficacy of cetuximab rechallenge.
The current results showing the efficacy of cetuximab rechallenge are different than those reported by Santini et al. who observed an RR of 54% with a median PFS of 6.6 months.9 Neither the study by Santini et al. nor the present study defined the inclusion criteria for the period between the date of the last cetuximab dose and the date of disease progression. In the present study, we collected the data at the end of enrolment to determine the causes for discontinuation of cetuximab as first-line treatment. In our cohort, the discontinuation was due to reasons other than progressive disease, such as adverse events in 5 patients (15%), indicating that these patients might have sensitivity to cetuximab. Although the study by Santini et al. did not report the causes for cetuximab discontinuation as first-line treatment, it remains possible that the rate of patients who discontinued cetuximab as first-line treatment due to reasons other than progressive disease was higher in their study compared with the present study. In the CRICKET trial, a Phase 2 study of cetuximab plus irinotecan rechallenge, the RR, DCR, median PFS and OS were 21%, 54%, 3.4 months and 9.8 months, respectively. Except for the RR, the efficacy outcomes were comparable between the CRICKET trial and the present study. One cause for the higher RR observed in the CRICKET trial might be due to the inclusion of patients with RAS and BRAF V600E wild-type mCRC, which was different from the present study.11 Therefore, a future Phase 3 study should enrol patients with RAS/BRAF wild-type mCRC. In addition, only left-sided mCRC patients should be eligible because previous studies have shown that right-sided mCRC patients had poor responses to anti-EGFR antibodies. In a Phase 2 study of panitumumab plus irinotecan rechallenge for patients with KRAS wild-type mCRC, the JACCRO CC-09, RR, DCR, median PFS and OS were 8%, 50%, 3.1 months and 8.9 months, respectively,12 suggesting that the efficacy outcomes of cetuximab rechallenge might be similar to those of panitumumab rechallenge.
During treatment with anti-EGFR antibodies, clonal evolution occurs, and tumour cells acquire gene alterations associated with resistance to anti-EGFR antibodies, such as KRAS, NRAS, EGFR, MET and ERBB2. In fact, in the CRICKET trial, 5 of the 6 patients who achieved PR did not have RAS mutations based on circulating tumour DNA (ctDNA) analysis just before the cetuximab rechallenge.11 These gene alterations associated with acquired resistance to anti-EGFR antibodies diminished and eventually disappeared with the lapse of time.10,13 This phenomenon provides further support to the superior efficacy of cetuximab rechallenge in patients with long CFIs compared to those with short CFIs observed in the present study. Therefore, CFI may be a surrogate marker for acquired gene alteration status. Although we did not conduct ctDNA analysis just before the cetuximab rechallenge to assess acquired resistance to anti-EGFR antibodies, gene alterations associated with acquired resistance to anti-EGFR antibodies might have disappeared in patients with long CFIs, and might have remained in patients with short CFIs. Therefore, these study results provide valuable information for clinical practice, especially in countries where ctDNA analysis has not yet been approved.
The frequency of skin toxicities associated with cetuximab was lower in the present study compared with the study by Santini et al., and the EPIC study in which patients received cetuximab for the first time.9,14 Although the underlying mechanism might be the shorter treatment duration in the present study compared with the other studies, this outcome suggests that cetuximab rechallenge might not increase the rate of cetuximab-associated adverse events.
The present study has several limitations. First, patients with KRAS exon 2 wild-type CRC were eligible. However, a few tumours with RAS or BRAF V600E mutations may have been included. Because only patients who achieved clinical benefit (complete, partial response or stable disease for ≥6 months) with first-line cetuximab-containing therapy were enrolled, we believe that most of the patients with RAS or BRAF V600E mutations were excluded from the present study. Second, the speed of tumour growth as natural history and efficacies of first-line cetuximab-containing treatment or doublet with or without bevacizumab in second-line treatment may affect CFI. However, the higher rate of DpR of > 0% in the long CFI group might indicate a treatment effect of cetuximab rechallenge rather than natural history. In addition, PFS of first-line doublet plus cetuximab and tumour shrinkage of second-line doublet with or without bevacizumab were not associated with the outcome of cetuximab rechallenge (data not shown). Third, it remains unclear whether the cut-off value of CFI defined by ROC analysis in this study was appropriate. Especially, because even though one responder had a short CFI according to the CFI cut-off value determined by ROC analysis, the patient’s CFI (338 days) was higher than the median CFI. Therefore, further validation studies are needed to confirm the appropriate value of CFI as a predictive marker for the efficacy of anti-EGFR antibody rechallenge.
Conclusion
This Phase 2 study showed that irinotecan plus cetuximab rechallenge as third-line treatment was safe and has promising activity in patients with KRAS wild-type mCRC who had a clinical response to cetuximab plus cytotoxic agents as first-line chemotherapy, warranting further investigation in a Phase 3 randomised trial. Specifically, patients with long CFIs might be good candidates for cetuximab rechallenge, whereas patients with short CFIs might not benefit from the cetuximab rechallenge.
References
Van Cutsem, E., Kohne, C. H., Hitre, E., Zaluski, J., Chang Chien, C. R., Makhson, A. et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med 360, 1408–1417 (2009).
Douillard, J. Y., Siena, S., Cassidy, J., Tabernero, J., Burkes, R., Barugel, M. et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J. Clin. Oncol. 28, 4697–4705 (2010).
Douillard, J. Y., Oliner, K. S., Siena, S., Tabernero, J., Burkes, R., Barugel, M. et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med 369, 1023–1034 (2013).
Cunningham, D., Humblet, Y., Siena, S., Khayat, D., Bleiberg, H., Santoro, A. et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med 351, 337–345 (2004).
Jonker, D. J., O’Callaghan, C. J., Karapetis, C. S., Zalcberg, J. R., Tu, D., Au, H. J. et al. Cetuximab for the treatment of colorectal cancer. N. Engl. J. Med 357, 2040–2048 (2007).
Van Cutsem, E., Peeters, M., Siena, S., Humblet, Y., Hendlisz, A., Neyns, B. et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J. Clin. Oncol. 25, 1658–1664 (2007).
Grothey, A., Cutsem, E. V., Sobrero, A., Siena, S., Falcone, A., Ychou, M. et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381, 303–312 (2013).
Mayer, R. J., Van Cutsem, E., Falcone, A., Yoshino, T., Garcia-Carbonero, R., Mizunuma, N. et al. A randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med 372, 1909–1919 (2015).
Santini, D., Vincenzi, B., Addeo, R., Garufi, C., Masi, G., Scartozzi, M. et al. Cetuximab rechallenge in metastatic colorectal cancer patients: how to come away from acquired resistance? Ann. Oncol. 23, 2313–2318 (2012).
Parseghian, C. M., Loree, J. M., Morris, V. K., Liu, X., Clifton, K. K., Napolitano, S. et al. Anti-EGFR-resistant clones decay exponentially after progression: implications for anti-EGFR re-challenge. Ann. Oncol. 30, 243–249 (2019).
Cremolini, C., Rossini, D., Dell’Aquila, E., Lonardi, S., Conca, E., Del Re, M. et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 5, 343–350 (2019).
Tsuji, A., Nakamura, M., Watanabe, T., Manaka, D., Matsuoka, H., Takeuchi, M. et al. Phase II study of third-line panitumumab rechallenge in patients with metastatic wild-type KRAS colorectal cancer who achieved a clinical benefit in response to first-line panitumumab plus chemotherapy. Ann. Oncol. 29, v68 (2018).
Siravegna, G., Mussolin, B., Buscarino, M., Corti, G., Cassingena, A., Crisafulli, G. et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med 21, 795–801 (2015).
Sobrero, A. F., Maurel, J., Fehrenbacher, L., Scheithauer, W., Abubakr, Y. A., Lutz, M. P. et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 2311–2319 (2008).
Acknowledgements
We thank the patients and their families, our collaborators who contributed to the study and recruited patients and the members of the JACCRO Data Center and Support Office. We thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Contributions
T.M. was involved in data acquisition, data analysis and interpretation and paper preparation. A.T. was involved in study concepts, quality control of data, data analysis and interpretation, paper editing and review. M.K. was involved in study design, data acquisition and paper review. M.N. was involved in study design, data acquisition and paper review. M.K., A.T., K.S. and T.D. were involved in study design and paper review. Y.S., H.T., H.H., T.S., T.W., T.T., Y.N., D.M., H.F., T.S. and M.T. were involved in data acquisition and paper review. W.I. was involved in study concepts, quality control of data, data analysis and interpretation, paper editing and review. M.F. was involved in study concepts, quality control of data, data analysis and interpretation and paper review.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
JACCRO institutional review board approved the present study (approval number: 2013-3). All patients provided written informed consent prior to participation. We conducted this study in accordance with the Declaration of Helsinki.
Consent to publish
All patients enrolled in the present study provided written informed consent for publication prior to participation.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
Toshiki Masuishi reports personal fees from Takeda, Chugai, Merck BioPharma, Taiho, Bayer, Lilly Japan, Yakult Honsha and Sanofi, grants from MSD, Daiichi Sankyo and Ono, outside the submitted work. Akihito Tsuji reports personal fees from Merck Serono Co., Ltd., during the conduct of the study, Taiho Pharmaceutical Co., Ltd., Eli Lilly Japan Co., Ltd., Chugai Pharmaceutical Co., Ltd., Sanofi Corporation and Bristol-Myers Squibb-Corporation, grants from Taiho Pharmaceutical Co., Ltd., Sanofi Corporation, Ono Pharmaceutical Co., Ltd., Pfizer Japan Inc, Kyowa Hakko Kirin Co., Ltd., Eisai Co., Ltd., MSD Corporation and Takeda Pharmaceutical Company Limited and grants from Toray Medical Co., Ltd. and Daiichi Sankyo Co., Ltd., outside the submitted work. Masahito Kotaka reports personal fees from Chugai Pharmaceutical and Yakult Honsya, Takeda Pharmaceutical, Merck Biopharma, Taiho Pharmaceutical, Lilly and Sanofi, outside the submitted work. Masato Nakamura reports personal fees from Merck, Yakult Honsha Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Takeda Pharmaceutical Company Limited, Taiho Pharmaceutical, Otsuka Pharmaceutical, Sanofi and ONO Pharmaceutical Co., Ltd., outside the submitted work. Yoshihiko Segawa reports personal fees from Japanese Society of Medical Oncology and Taiho Pharmaceutical Co., Ltd., grants from Kindai University, Taiho Pharmaceutical Co., Ltd., Novartis Pharma K.K. and Eisai Co., Ltd., outside the submitted work. Hiroki Hara reports grants from Astrazeneca, grants and personal fees from Daiichi Sankyo, grants from Dainippon Sumitomo Pharma, personal fees from Lilly, grants from Merck Biopharma, grants and personal fees from MSD, Taiho and Chugai, grants from Eisai, LSK BioPharma, Incyte, Pfizer, Boehringer-Ingelheim and Beigene, grants and personal fees from ONO and BMS, personal fees from Yakult Honsha, Sanofi and Takeda, grants from Astellas and personal fees from Kyowa Hakko Kirin, outside the submitted work. Takao Takahashi reports grants from Yakult Honsha Co., Ltd., during the conduct of the study, personal fees from Takeda Pharmaceutical Co., Ltd. and Sanofi Co., Ltd., outside the submitted work. Wataru Ichikawa reports grants and personal fees from Chugai Pharma, Taiho Pharmaceutical and Takeda, personal fees from Daiichi Sankyo, grants and personal fees from Merck Serono, personal fees from Yakult and Bayer Yakuhin, grants from Eizai and Shionogi and grants and personal fees from Ono Pharmaceutical, outside the submitted work. Mitsugu Kochi, Akinori Takagane, Ken Shimada, Tadamichi Denda, Hiroaki Tanioka, Tamotsu Sagawa, Takanori Watanabe, Yuji Negoro, Dai Manaka, Hideto Fujita, Takeshi Suto, Masahiro Takeuchi, Masashi Fujii report declaration of interest: none.
Funding information
Japan Clinical Cancer Research Organization (JACCRO).
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41416_2020_1042_MOESM4_ESM.xlsx
Supplementary Table 1. Median progression-free survival and overall survival with hazard ratios using various cut-off values for cetuximab-free interval
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Masuishi, T., Tsuji, A., Kotaka, M. et al. Phase 2 study of irinotecan plus cetuximab rechallenge as third-line treatment in KRAS wild-type metastatic colorectal cancer: JACCRO CC-08. Br J Cancer 123, 1490–1495 (2020). https://doi.org/10.1038/s41416-020-01042-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-01042-w
This article is cited by
-
Therapeutic landscape and future direction of metastatic colorectal cancer
Nature Reviews Gastroenterology & Hepatology (2023)
-
Phase II Study of Third-Line Panitumumab Rechallenge in Patients with Metastatic Wild-Type KRAS Colorectal Cancer Who Obtained Clinical Benefit from First-Line Panitumumab-Based Chemotherapy: JACCRO CC-09
Targeted Oncology (2021)