Abstract
Osimertinib is an irreversible, third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor that is highly selective for EGFR-activating mutations as well as the EGFR T790M mutation in patients with advanced non-small cell lung cancer (NSCLC) with EGFR oncogene addiction. Despite the documented efficacy of osimertinib in first- and second-line settings, patients inevitably develop resistance, with no further clear-cut therapeutic options to date other than chemotherapy and locally ablative therapy for selected individuals. On account of the high degree of tumour heterogeneity and adaptive cellular signalling pathways in NSCLC, the acquired osimertinib resistance is highly heterogeneous, encompassing EGFR-dependent as well as EGFR-independent mechanisms. Furthermore, data from repeat plasma genotyping analyses have highlighted differences in the frequency and preponderance of resistance mechanisms when osimertinib is administered in a front-line versus second-line setting, underlying the discrepancies in selection pressure and clonal evolution. This review summarises the molecular mechanisms of resistance to osimertinib in patients with advanced EGFR-mutated NSCLC, including MET/HER2 amplification, activation of the RAS–mitogen-activated protein kinase (MAPK) or RAS–phosphatidylinositol 3-kinase (PI3K) pathways, novel fusion events and histological/phenotypic transformation, as well as discussing the current evidence regarding potential new approaches to counteract osimertinib resistance.
Similar content being viewed by others
Background
The identification of activating mutations in the gene encoding the epidermal growth factor receptor (EGFR) tyrosine kinase in patients with non-small cell lung cancer (NSCLC) and the subsequent development of targeted therapy with small-molecule EGFR tyrosine kinase inhibitors (TKIs) has dramatically revolutionised the treatment landscape of these tumours. Somatic, activating mutations in the tyrosine kinase domain of EGFR are present in about 15% of Caucasian and nearly 50% of Asian patients with advanced NSCLC.1,2 Almost 90% of these mutations consist of deletions in exon 19 or L858R point mutations within exon 21. These genetic changes act as oncogenic drivers leading to ligand-independent activation of EGFR downstream signalling, thus promoting cell proliferation, survival and migration.
Several large-scale Phase 3 clinical trials have consistently demonstrated the superior efficacy of first-generation (gefitinib and erlotinib) and second-generation (afatinib) TKIs in comparison with standard first-line platinum-based chemotherapy for the treatment of patients with advanced NSCLC with activating EGFR mutations.3 Unfortunately, despite a remarkably high (60–70%) objective response rate (ORR) to such treatments, the majority of patients develop resistance, with average progression-free survival (PFS) ranging from 9 to 15 months.3 The most common resistance mechanism results from the development of the so-called ‘gatekeeper’ T790M mutation in EGFR exon 20, which sterically hinders the binding of first- and second-generation TKIs to the ATP-binding site of EGFR.4 However, this limitation has been overcome by the introduction of third-generation TKIs, particularly osimertinib. Osimertinib (Tagrisso™, [AZD9291] AstraZeneca, Cambridge, UK) is an orally administered EGFR-TKI that selectively targets activating EGFR mutations, as well as the T790M-resistance mutation, through the formation of a covalent bond to the C797 residue in the ATP-binding site of mutant EGFR. Compared with first- and second-generation EGFR-TKIs, osimertinib demonstrated a superior activity against T790M mutants in vitro, with minimal off-target effects resulting, when tested in patients, in fewer adverse events usually associated with the blockade of wild-type (wt) EGFR.5 Currently, osimertinib is the only third-generation EGFR-TKI approved by major regulatory agencies for treatment of T790M-positive patients who have progressed on first- or second-generation EGFR-TKIs. Osimertinib was also approved in 2018 as first-line therapy for advanced EGFR-mutated NSCLC, regardless of T790M mutation status.6 However, despite the robust clinical activity exerted by osimertinib, patients inevitably develop secondary resistance to this treatment, which poses a significant challenge due to the paucity of post-osimertinib pharmacological options available to date. The aim of this review is to provide a comprehensive overview of resistance mechanisms to osimertinib and to take advantage of the available knowledge to build future strategies and gain insights to overcome resistance to this agent.
Osimertinib therapy
Osimertinib treatment after failure of previous EGFR-TKI therapy
The clinical efficacy of osimertinib for the treatment of patients with advanced EGFR-mutated NSCLC who had experienced disease progression during prior therapy with EGFR-TKIs was first documented in the international Phase 1/2 AURA trial.7 In this study, osimertinib achieved an ORR of 61% (95% confidence interval [CI]: 52–70) among patients with centrally confirmed T790M mutations (n = 127). In the same population, median PFS was 9.6 months (95% CI: 8.3 to not reached) and the recommended dose for future trials was set at 80 mg/day.7 The subsequent AURA Phase 2 extension study and the open-label Phase 2 AURA2 trial confirmed the safety and efficacy of osimertinib as a post-EGFR-TKI treatment in patients with EGFR-mutated NSCLC with the T790M mutation.8,9
Osimertinib was further investigated in the randomised international Phase 3 AURA3 trial, which compared this compound with platinum–pemetrexed combination chemotherapy in EGFR T790M-positive NSCLC patients following progression during prior EGFR-TKI treatment.10 The median PFS for patients treated with osimertinib was more than twice that of those treated with platinum-based chemotherapy (10.1 versus 4.4 months, hazard ratio [HR]: 0.30; 95% CI: 0.23–0.41, P < 0.001). In addition, ORR for osimertinib was 71% compared with 31% for platinum-based chemotherapy (P < 0.001). Remarkably, the treatment with osimertinib was also well tolerated, with a lower incidence of grade 3 adverse effects than the chemotherapy arm (23% versus 47%).10 The long-term follow-up data from a pre-planned pooled analysis of AURA and AURA2 trials confirmed the efficacy of osimertinib in T790M-positive EGFR-TKI pre-treated patients.11 The median overall survival (OS) was 26.8 months (95% CI: 24.0–29.1 months) and the 12-month survival rate was 80%.11 These striking results led to the Food and Drug Administration (FDA) approval of osimertinib for the treatment of patients with metastatic EGFR-mutated NSCLC with an acquired T790M mutation after progression on previous EGFR-TKI therapy.
Osimertinib as first-line therapy
Following the impressive clinical results shown by osimertinib as a salvage therapy in the presence of the T790M secondary mutation, first-line treatment with osimertinib in an attempt to avoid this acquired resistance was investigated.6,12 The FLAURA Phase 3 trial directly compared the efficacy and safety of osimertinib with standard first-generation EGFR-TKIs (gefitinib or erlotinib) in 556 previously untreated patients with advanced, EGFR mutation-positive NSCLC.6 In this randomised, double-blind study, all the enrolled patients had exon 19 deletions or L858R mutations in their baseline tumour tissue samples. The primary endpoint, investigator-assessed PFS, was significantly longer following osimertinib treatment compared with treatment with first-generation EGFR-TKIs (18.9 versus 10.2 months, HR: 0.46, 95% CI: 0.37–0.57; P < 0.0001). No significant difference was reported in terms of ORR (80% versus 76% for osimertinib and standard EGFR-TKI therapy, respectively, odds ratio [OR]: 1.27; 95% CI: 0.85–1.90; P = 0.24), but at interim analysis, although only 25% mature, an early trend towards a better OS was shown, with a 37% reduction in the risk of death following treatment with osimertinib versus standard EGFR-TKI therapy (HR: 0.63; 95% CI: 0.45–0.88, P = 0.0068). Moreover, osimertinib was better tolerated, with fewer grade 3 or higher adverse effects when compared with first-generation EGFR-TKIs (34% versus 45%).6 In view of these results, osimertinib has been propelled into first-line therapy for patients with advanced or metastatic NSCLC with activating EGFR mutations, regardless of their T790M status.13 Despite these results, the possibility of sequencing treatment with first-/second-generation EGFR-TKIs followed by osimertinib could be a potential alternative with consistent data but applicable only in patients developing T790M mutation.3
Osimertinib efficacy in central nervous system metastases
Central nervous system (CNS) metastases are a common poor prognostic factor in patients with advanced NSCLC with EGFR mutations, occurring in approximately 30% of patients during treatment with an EGFR-TKI.14 Osimertinib has shown excellent CNS penetration in preclinical studies as well as in clinical trials both as a first- and second-line therapy in patients with EGFR-mutant NSCLC.15,16 The CNS efficacy of second-line osimertinib was assessed in a subgroup of patients with stable, asymptomatic brain metastases within the Phase 3 AURA3 study.17 Median CNS PFS was 11.7 months for osimertinib compared with 5.6 months for chemotherapy (HR: 0.32; 95% CI: 0.15–0.69; P = 0.004), and responses were also durable.17 At the point of data cut-off (15 April 2016), CNS ORR in patients with one or more measurable CNS lesions was 70 and 31%, in response to treatment with osimertinib and platinum–pemetrexed, respectively (OR: 5.13; 95% CI: 1.44–20.64; P = 0.015).17
The Phase 3 FLAURA study also revealed the high CNS efficacy of osimertinib in the first-line setting.18 In patients with documented CNS metastases, median CNS PFS was 13.9 months (95% CI: 8.3 months to not calculable) with standard EGFR-TKIs but was not reached with osimertinib (95% CI: 16.5 months to not calculable) (HR: 0.48; 95% CI: 0.26–0.86; P = 0.014). Remarkably, fewer patients in the osimertinib arm developed new brain lesions compared with the control arm (12% versus 30%), supporting the protective role of osimertinib in the development of new CNS lesions.18 Osimertinib also demonstrated an encouraging activity in leptomeningeal metastases (LM), both in first-line18 and in second-line19 settings. Taken together, these findings further corroborate the use of osimertinib for the front-line treatment of patients with EGFR-mutated NSCLC.
Resistance to osimertinib
Despite the success of osimertinib both in the first-line treatment setting and as a salvage therapy in the presence of the T790M secondary mutation, acquired resistance inevitably occurs—similar to patients treated with first- or second-generation EGFR-TKIs—thus limiting a prolonged clinical benefit achieved with this compound. Moreover, a fraction of patients can experience intrinsic resistance to osimertinib, as observed following treatment with other EGFR-TKIs.
The molecular heterogeneity of NSCLC substantially influences the possible mechanisms of resistance to osimertinib, contributing to the wide spectrum of resistance aberrations discovered so far. In addition, multiple co-existing molecular alterations have been observed in a considerable percentage of patients, both when osimertinib was administered as a front-line therapy as well as after the failure of previous EGFR inhibitors. Hence, repeated tumour biopsies as well as plasma genotyping at the time of progression on osimertinib are crucial steps in unravelling resistance mechanisms and guiding future treatments.
Acquired resistance mechanisms to EGFR-TKIs can be broadly grouped into EGFR-dependent or EGFR-independent mechanisms. Some of the mechanisms are overlapping when osimertinib is administered as a first- or second-line therapy, whereas others have been identified only in one of these settings (Fig. 1). What follows is a description of our current knowledge about the emerging resistance mechanisms to osimertinib in patients with EGFR-mutated NSCLC. A schematic representation of the main resistance mechanisms to osimertinib is provided in Fig. 2.
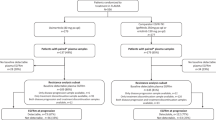
Resistance mechanisms reported for osimertinib according to the line of treatment. The two pie charts depict resistance mechanisms that have been identified in tissue and/or in plasma after resistance to second-line and first-line osimertinib, respectively. Only studies that enrolled more than 15 patients have been taken into account for the ranges of the percentages. In some cases, different molecular aberrations might co-exist in the same patient
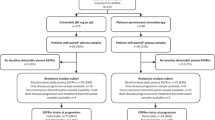
Schematic representation of the known mechanisms of resistance to osimertinib Resistance mechanisms to osimertinib can consist of EGFR modifications (mutation/amplification), bypass pathway activation, downstream pathway activation, epithelial-to-mesenchymal transition (EMT), histologic transformation, oncogenic gene fusions and cell-cycle gene aberrations. Abbreviations: act, activation; amp, amplification; del, deletion; mut, mutation
EGFR-dependent mechanisms of resistance
Insights into EGFR-dependent resistance mechanisms to second-line osimertinib therapy have come from the circulating tumour DNA (ctDNA) genomic profile conducted using plasma from patients with T790M-positive NSCLC enrolled in the AURA3 trial.20 In this study, acquired EGFR mutations (most commonly C797S; 14%) were observed in 21% of the samples, and all patients with acquired tertiary EGFR mutations retained the T790M mutation after progression on osimertinib.20 However, 49% of patients lost T790M mutation at the time of progression on osimertinib,20 a percentage that was consistent with previous findings.21,22,23 Among the samples that showed a loss of the T790M mutation, ex19del was preferentially present compared with the L858R mutation (83% versus 14%, respectively).20 Loss of the T790M mutation manifests as resistance to second-line osimertinib and is usually associated with the emergence of competing resistance mechanisms such as KRAS mutations, MET amplification, small-cell transformation and gene fusions20 (see below). When Oxnard and collaborators21 performed next-generation sequencing on tumour biopsy samples after the development of acquired osimertinib resistance in 143 patients who received second-line osimertinib for T790M-positive advanced EGFR-mutant NSCLC, they found that the loss of T790M is usually associated with early resistance to osimertinib and a shorter time to treatment discontinuation (6.1 versus 15.2 months).21 Further studies confirmed the detrimental impact of T790M loss on patients’ PFS and OS.20,22,24 It has also been proposed that the timing of the emergence of osimertinib resistance can shed light on the molecular mechanism, with early resistance usually associated with the loss of the T790M mutation, and late resistance linked to retention of T790M.21 In addition, plasma levels of EGFR T790M and activating mutations could predict the type of acquired resistance mechanisms.21
As expected, when looking at the next-generation sequencing analysis conducted on plasma samples from 91 patients who received front-line osimertinib therapy in the FLAURA trial, no evidence of emergence of the T790M mutation in the osimertinib arm was found. These data were concordant with the pharmacodynamic activity of osimertinib: considering that osimertinib is selective for both EGFR-sensitising and T790M mutations, the emergence of T790M under osimertinib treatment was not expected to be a resistance mechanism.25
Other EGFR-dependent mechanisms of resistance to osimertinib include the development of EGFR tertiary mutations or amplifications,26,27 which are more likely to arise in cases in which the EGFR T790M mutation is retained.20,21,23
Mutations in C797
The most common tertiary EGFR mutation is EGFR C797S, which occurs in exon 20 and accounts for 10–26% of cases of resistance to second-line osimertinib treatment.10 When osimertinib was administered as a front-line treatment, the frequency of the C797S mutation was 7%, making it the second most frequent mechanism, behind MET amplification, of drug resistance in this setting.25 The EGFR C797S mutation, in which cysteine at codon 797 within the ATP-binding site is substituted for by serine, results in the loss of the covalent bond between osimertinib and the mutant EGFR. Predictably, the C797S mutation also confers cross-resistance to other irreversible third-generation TKIs, including rociletinib, olmutinib and narzatinib, by preventing their binding to the EGFR active site.26,28,29
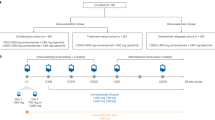
Importantly, the allelic context in which C797S is acquired has potential implications for treatment (Fig. 3). Given that C797S-positive cells are still sensitive to quinazoline-based EGFR-TKIs, the rare eventuality of the emergence of C797S in trans with the T790M mutation allows cells to be targeted with both first-generation and third-generation EGFR-TKIs in order to hit C797S- and T790M-positive alleles, respectively. On the other hand, when the mutations are in cis, the cells are found to be resistant to all available EGFR-TKIs alone as well as combined.28,30,31 On the basis of preclinical data, clinical efficacy—although limited—of this therapeutic strategy has been reported.30,31
Potential treatment algorithm of T790M EGFR-mutated NSCLC. The present figure depicts the most common molecular events within EGFR that can occur after the onset of osimertinib resistance. (1) T790M loss/C797S: in this scenario, NSCLC cells re-acquire sensitivity to first-generation and second-generation EGFR-TKIs; (2) T790M/C797S in cis: NSCLC cells are amenable to novel fourth-generation EGFR-TKIs which are currently in development and (3) T790M/C797S in trans: NSCLC cells become sensitive to combination therapy with osimertinib and first-generation EGFR-TKIs
Yang and collaborators26 investigated the mutation profile of plasma samples obtained from 93 patients with advanced NSCLC treated with osimertinib as a second-line therapy. The authors identified tertiary EGFR mutations conferring osimertinib resistance in one-third of the patients. Of these mutations, 24% were the well-known C797S substitution, with co-existing C797G mutation noted in two cases.26 The novel tertiary EGFR C797G mutation was detected by next-generation sequencing on a pleural biopsy specimen of a single patient who progressed on second-line osimertinib, and it was concomitant with MYC and EGFR amplifications (see below).32
Mutations in G796
Besides C797X mutations, a number of other rare point mutations in EGFR have been identified. On the basis of protein structure prediction, solvent-front mutations in the G796 residue—G796R, G796S and G796D—which is adjacent to C797, have the potential to sterically interfere with the osimertinib–EGFR interaction. G796R has a major impact while G796S has a milder impact on osimertinib–EGFR binding.26,33,34 In addition, a G796D mutation has also been detected in a single patient who developed second-line osimertinib resistance.35
Mutations in L792, L718 and G719
The EGFR protein kinase domain has a NH2-terminal lobe (N-lobe) and a larger COOH-terminal lobe (C-lobe) connected by the so-called ‘hinge’ region of the kinase. In silico modelling has demonstrated that mutations in the L792 residue in this region can sterically interfere with a methoxy group on the phenyl ring of osimertinib and disrupt its binding to the kinase domain.34 A variety of hinge-pocket mutations in the L792 residue have been reported, among which L792H is the most common.26 Interestingly, L792 mutations usually co-exist with other EGFR mutations and occur in cis with T790M but in trans with G796/C797, when present simultaneously in the same patient.26 Moreover, L792 mutant variants remained sensitive to gefitinib in vitro.26 Mutations in the L718 residue are also responsible for osimertinib resistance in vitro and in vivo.26,36 Most of them are L718Q, and the majority of patients with L718 mutations do not have co-existing C797 mutations, suggesting that these mutations could lead independently to osimertinib resistance. The L718 residue is located in the ATP-binding site of the EGFR kinase domain, and in silico modelling has shown that a substitution in this residue can cause spatial restriction and hinder the binding of osimertinib to EGFR. Steric restriction as predicted by in silico modelling might also be the cause of osimertinib resistance in patients with G719A mutations, owing to the close proximity of the G719A residue to the L718 residue.26,34 However, in vitro studies have also demonstrated that L718Q might still be sensitive to first- and second-generation EGFR-TKIs, especially when T790M is lost,37 and this finding has been confirmed in the clinical setting.23 Of note, EGFR L718Q has been also found to be a resistance mechanism to first-line osimertinib treatment, accounting for 2% of cases.25
Mutations in G724
G724S mutation in the P-loop of the EGFR kinase domain has been identified after progression to osimertinib in multiple patient cases.38,39,40,41,42 Structural analyses demonstrated that G724S mutation induces a conformational change in the receptor which impairs the binding of osimertinib.39 Even though the incidence of this rare mutation upon osimertinib treatment is unknown yet, due to the relatively limited evidence available, it is supposed that G724S-mediated resistance preferentially occurs in ex19del but not L858R thus acting in an allele-specific manner.43 Interestingly, second-generation EGFR-TKIs retain kinase affinity in G724S mutants, and afatinib was successful in overcoming G724S-mediated resistance to osimertinib in vitro.43
Mutations in exon 20
Besides well-known EGFR tertiary mutations ascribed as responsible for osimertinib resistance, other mutations within exon 20 can infrequently occur after progression to osimertinib, but their role in mediating resistance is not established yet.
EGFR S768I constitutes a rare mutation in exon 20 that can be found in conjunction with sensitizing EGFR mutations at the beginning of EGFR-TKI treatment (occurring in <1% cases).44 Even though the exact prognostic and predictive role of S768I is not fully clarified due to its rarity, it has been detected in patients treated with osimertinib in second-line42 and also in one patient after progression on front-line osimertinib treatment.25
Exon 20 insertion has also been reported in one patient after failure of second-line osimertinib therapy (1%).20
EGFR gene amplification
In the second-line setting, amplification of the wt EGFR allele in addition to the presence of the EGFR-ex19del allele constituted a novel mechanism of resistance.45,46,47 A study by Kim and colleagues48 also revealed increased EGF mRNA expression in tumour tissue samples of patients who progressed on osimertinib.
EGFR-independent mechanisms of resistance
Osimertinib resistance mediated by EGFR-independent mechanisms can be acquired by activation of alternative bypass pathways, aberrant downstream signalling or histologic transformation. Importantly, these aberrations can co-occur within the same tumour and co-exist with EGFR tertiary mutations, underlying the complexity and heterogeneity of cancer evolution in response to EGFR-TKI treatment.
MET amplification
MET gene amplification constitutes the most frequent cause of bypass pathway activation as an acquired resistance mechanism to EGFR-TKIs.20,49,50 This event leads to osimertinib resistance by persistent activation of signalling pathways downstream of EGFR, such as those mediated by MAPK, signal transduction and activator of transcription (STAT) and phosphatidylinositol 3-kinase (PI3K)–Akt, independent of EGFR activation and signalling. MET amplification can occur with or without loss of the T790M mutation, when osimertinib is given after failure of previous EGFR-TKIs.23,51,52,53,54 In this clinical setting, the loss of T790M was experienced by 43% of patients, whereas 57% retained the mutation.20 In the plasma next-generation sequencing analysis conducted within the AURA3 study, MET amplification was observed in nearly 19% of the samples at disease progression and/or treatment discontinuation.20 Remarkably, MET amplification co-occurred with EGFR C797S in 7% of cases,20 and is also likely to be associated with CDK6 and BRAF amplifications.23 Due to the relative proximity of CDK6, MET and BRAF on chromosome 7q (7q21.2, 7q31.2 and 7q34, respectively), a single genomic event could be hypothesised to be responsible for gene amplifications.23 When osimertinib was given as a first-line therapy, MET amplification was the most common resistance mechanism, encountered in 15% of patients by next-generation sequence ctDNA analysis.25 Moreover, this percentage is expected to be higher in tissue, due to the underestimation of gene amplification in plasma.25 MET amplification could also represent a potential mechanism of intrinsic resistance to osimertinib.54,55
Consistent with these findings, several preclinical studies have demonstrated that the concomitant use of c-Met inhibitors, such as crizotinib, with osimertinib has the potential to overcome resistance in osimertinib-resistant EGFR-mutant NSCLC cell lines with MET gene amplification.23,51,54 Moving to the clinic, the combination of crizotinib and osimertinib could be an effective therapeutic strategy in the eventuality of MET amplification onset at the time of acquired resistance to osimertinib.56,57,58 The same drug combination reversed MET exon 14 skipping-mediated resistance to osimertinib in EGFR-mutated NSCLC cells.59 In the clinical setting, MET exon 14 skipping has been recently reported in one patient after failure of second-line osimertinib.42 Yang et al.26 have also identified rare mutations of MET—P97Q and I865F—in ctDNA in two cases of second-line osimertinib resistance, but their significance is unknown so far.
HER2 amplification
ErbB2 (encoded by HER2) is a tyrosine kinase receptor that belongs to the EGFR family and activates the downstream PI3K–Akt and MAPK/extracellular signal-regulated kinase (ERK) kinase (MEK)–ERK/MAPK pathways. HER2 amplification has been identified in 5% of patients who have acquired resistance to second-line osimertinib,20 and it was earlier thought to be mutually exclusive with the T790M mutation, as described for first-generation TKIs.60,61 Moreover, HER2 amplification can co-exist with EGFR L792X + C797X + PIK3CA amplification (in 1% of cases), EGFR G796S + MET amplification (1%), and PIK3CA amplifications (1%).20 Moving to acquired resistance to first-line osimertinib therapy, HER2 amplification was detected in 2% of cases.25 More recently, HER2 amplification has been reported in one patient who experienced intrinsic resistance to osimertinib.55 From the preclinical point of view, Ortiz-Cuaran et al.54 studied the impact of HER2 overexpression on sensitivity to osimertinib and rociletinib in the PC9GR cell line (T790M positive, EGFR-mutant) and found that HER2 overexpression decreased sensitivity to both drugs.
RAS–MAPK pathway activation
RAS–MAPK pathway aberrations are known to lead to osimertinib resistance in patients with EGFR-mutated NSCLC. Eberlain et al.62 identified NRAS mutations, including the novel E63K mutation, in osimertinib-resistant EGFR-mutant NSCLC cell lines together with a gain of copy number of wt NRAS or wt KRAS in cell populations resistant to gefitinib, afatinib, WZ4002 or osimertinib. In addition, the combination of osimertinib with the MEK inhibitor selumetinib was found to prevent EGFR-TKI resistance both in vitro and in vivo.62 Ortiz-Cuaran et al.54 identified a KRAS G12S mutation on tumour re-biopsy at the onset of acquired resistance to second-line osimertinib. Other KRAS mutations, such as G13D, Q61R and Q61K, have also been identified.21,23,45 KRAS G12D has been reported after osimertinib failure both in first-line and in subsequent lines of therapy,20,25,47,63 and pre-existing KRAS G12D mutations in association with PTEN loss were also detected in two patients who showed primary resistance to second-line osimertinib.63
Within the RAS–MAPK pathway, the BRAF V600E mutation has been identified as being responsible for osimertinib resistance in 3% of cases,20,25 both in first- and in second-line therapy, in association22,64 or not65 with the EGFR T790M mutation when osimertinib was administered after failure of previous EGFR-TKIs. Two cases of concurrent BRAF V600E mutation and MET amplification as a resistance mechanism to first-line osimertinib therapy have also been reported.25,66 Remarkably, cell lines that harboured BRAF V600E as a resistance mechanism to osimertinib showed sensitivity to a combination of encorafenib (a BRAF inhibitor) and osimertinib.64 Additionally, Kim et al.48 have reported MAPK1 mRNA overexpression in a patient treated with osimertinib in second-line therapy at the time of progression.
PI3K pathway activation
Bypass activation of the PI3K pathway can occur both via PIK3CA mutation/amplification and PTEN deletion.48,67 Contrary to the mutual exclusivity of most oncogenic driver mutations, the co-occurrence of PIK3CA mutations with mutations in other oncogenic driver genes is well described in NSCLC,68 in agreement with the supporting role of PIK3CA to several oncogenic signalling in NSCLC.69 However, although concurrent PIK3CA mutations are poor prognostic factors, no significant differences were found in clinical outcomes in patients affected by concurrent PIK3CA and EGFR mutations treated with EGFR-TKI monotherapy, promoting the use of EGFR-TKIs in such patients.68 Among the known PIK3CA mutations, E545K, E542K, R88Q, N345K and E418K, which occur at a frequency of 4–11%, have been described to confer resistance to second-line osimertinib therapy.20,21,23,47,63 The role of PIK3CA E545K in mediating osimertinib resistance has been confirmed in vitro.23 Of note, in the next-generation sequencing analysis performed within the AURA3 study, PIK3CA amplifications co-occurred with HER2 amplifications in two out of three cases.20 Among patients treated with frontline osimertinib, the PIK3CA mutations E453K, E545K and H1047R were identified in six cases, with E545K being the most represented (4%).25
Cell-cycle gene alterations
Alterations in the genes encoding cell-cycle proteins have been found in 12 and 10% of patients who progressed on osimertinib administered as a second-line and first-line therapy, respectively,20,25 and have been reported to be associated with poor outcomes following osimertinib treatment.70 In particular, several studies have reported amplifications in the genes encoding cyclin D1, cyclin D2, cyclin E1, cyclin-dependent kinase (CDK) 4 and CDK6, and a frameshift deletion in the gene encoding the CDK inhibitor 2A.20,25
Oncogenic fusions
Oncogenic fusions, which are likely to behave as oncogenic drivers, have been identified in 3–10% of cases of acquired resistance to second-line osimertinib, and they can co-occur with EGFR C797S, BRAF mutation and MET amplification.20 They include FGFR3–TACC3 and RET–ERC1,20 with CCDC6–RET, NTRK1–TPM3, NCOA4–RET, GOPC-ROS1, AGK–BRAF and ESYT2–BRAF also identified as fusions that are potentially responsible for acquired resistance to second-line osimertinib.21,23,71,72 The product of the oncogenic fusion SPTBN1–ALK has been detected in one patient who received front-line osimertinib but not in patients treated with second-line.20,25 Notably, combined EGFR and Ret inhibition with osimertinib and BLU-667 was effective at overcoming resistance due to the presence of the CCDC6–RET fusion.71 Another study identified receptor tyrosine kinase fusions and BRAF kinase fusions as rare events that mediated resistance to TKIs in patients with advanced NSCLC, including a novel PLEKHA7–ALK fusion following osimertinib treatment.73 Lastly, an acquired EML4-ALK fusion has been confirmed after progression on second-line osimertinib; in this case, the addition of crizotinib to osimertinib led to stability of disease.74
Histologic and phenotypic transformation
Histologic transformation from NSCLC to small-cell lung cancer (SCLC), which arises in 4–15% of cases, is a known mechanism of resistance to first-generation EGFR-TKIs that dramatically impacts patients’ prognosis, and has been reported as an important cause of resistance to osimertinib.75,76,77,78,79 A comprehensive understanding of the underlying mechanism(s) responsible for the histologic transformation is still missing. Lee and colleagues80 performed whole-genome sequencing of serially acquired biopsy samples from four patients who underwent SCLC transformation under EGFR-TKI treatment, to study the clonal evolution and genetic mechanisms contributing to the event. The authors found complete inactivation of the tumour-suppressor genes RB1 and TP53 in the initial NSCLC, as well as at the time of SCLC transformation, pointing to inactivated RB1 and TP53 as potential predisposing factors for histologic transformation.80 Thus, NSCLC patients who harbour inactivated RB1 and TP53 may deserve monitoring for transformation into SCLC during their clinical history.80,81 Focusing on SCLC transformation under osimertinib treatment, RB1 and TP53 are likely to be inactive at the time of this event, as documented in the case of first-generation and second-generation EGFR-TKIs.75 Moreover, the assessment of RB1 and TP53 mutational status on ctDNA, as well as the test of neuron-specific enolase (NSE) levels in plasma at the time of progression on osimertinib could be taken into account to unravel a potential SCLC transformation.75 Notably, SCLC transformation has been ascribed as a putative mechanism of primary resistance to osimertinib.75 On the basis of the limited evidence available to date, in the case of SCLC transformation, a favourable response to combination chemotherapy with platinum and etoposide can be envisioned.77 More recently, histologic transformation towards squamous cell carcinoma has been observed after failure of both first-line and second-line osimertinib.42
Regarding epithelial-to-mesenchymal transition (EMT), it has been reported that cells from patients with NSCLC with acquired resistance to gefitinib or osimertinib exhibit EMT features, with a decrease in epithelial cell junction proteins such as E-cadherin and an increase in mesenchymal markers such as vimentin, without acquiring any secondary EGFR mutations.82,83 The EMT features of osimertinib-resistant cells were related to the upregulation of the zinc finger transcription factor Zeb1, and reversing EMT by the dual histone deacetylase (HDAC) and hydroxy-3-methylglutaryl co-enzyme A reductase (HMGR) inhibitor JMF3086 was successful in restoring sensitivity to osimertinib in vitro.82 Resistance to osimertinib has also been associated with overexpression of the EMT transcription factor TWIST-1 in EGFR-mutated NSCLC cells, and novel strategies to counteract this event using TWIST-1 inhibitors are currently being investigated.84
Other mechanisms
Focal amplification of the FGFR1 gene accompanied by nearly 20-fold higher levels of fibroblast growth factor 2 (FGF2) mRNA compared with baseline has been reported in an osimertinib-resistant patient.48 The co-existence of FGFR1 amplification and increased levels of FGF2, which is a FGFR1 (FGF receptor 1) ligand, suggests an autocrine loop-mediated mechanism of resistance.48 In addition, one case of FGFR3/FGFR19 amplification has been described.23
Src family kinases (SFKs) and focal adhesion kinase (FAK) play a critical role in sustaining the Akt and MAPK pathways under EGFR inhibition in osimertinib-sensitive cells.83 The amplification of the SFK member YES1 has been reported as a resistance mechanism to osimertinib in NSCLC cell lines, and inhibiting SFK or FAK signalling enhanced the effects of osimertinib in vitro.83 Another emerging key player involved in osimertinib intrinsic resistance is the receptor tyrosine kinase Anexelekto (AXL).85 AXL can interact with other tyrosine kinase receptors, including EGFR and HER3, and sustain survival of tumour cells exposed to osimertinib.85 In a recent research study, AXL overexpression was associated with a poor response to osimertinib, whereas combination treatment with an AXL inhibitor and osimertinib prevented the development of intrinsic resistance to osimertinib and the subsequent emergence of drug-tolerant clones in vitro and in vivo.85
Therapeutic strategies to overcome osimertinib resistance
Despite the promising results obtained with osimertinib in advanced patients with NSCLC, resistance ultimately develops due to the treatment selection pressure and the inherent heterogeneity of NSCLC. The intra-patient heterogeneity, and co-occurrence, of multiple resistance mechanisms constitute a major challenge in developing an efficient treatment strategy to counteract tumour progression. Remarkably, the clonal evolution of oncogene-addicted NSCLC can give rise to different molecular aberrations both in space (between primary tumour and metastasis) and in time (after treatment failure), contributing to the complexity of the molecular resistance machinery.86
The loss of the T790M mutation during osimertinib therapy is usually associated with treatment failure. Under this circumstance, in the presence of the original driver EGFR mutation, it might be feasible to re-treat patients with first-generation EGFR-TKIs.87 However, as reported by other authors, the loss of T790M might be accompanied by the emergence of alternative resistance mechanisms such as MET amplification and KRAS mutation — situations in which first-generation EGFR-TKIs are not effective.21
Given that EGFR C797S is the most common tertiary mutation in patients with T790M-positive osimertinib-resistant NSCLC, overcoming this mutation is the focus of many studies. To this end, fourth-generation EGFR-TKIs have been developed in order to successfully target the C797S mutant EGFR.88 Among these, EAI045 is an EGFR allosteric inhibitor which has shown efficacy against C797S-T790M-L858R triple mutant cells when given in combination with the anti-EGFR antibody cetuximab.89,90 However, it is not effective against C796S-T790M-ex19del triple mutant cells due to its different structure at the allosteric pocket compared with the C797S-T790M-L858R variant.89 JBJ-04-125-02—a novel EGFR allosteric inhibitor—has been recently found to inhibit EGFR C797S-T790M-L858R signalling in vitro and in vivo, and the combination of JBJ-04-125-02 with osimertinib was more effective than either single agent alone.91 Brigatinib, a novel dual-target ALK-EGFR inhibitor, has been found to be effective against C797S-T790M-ex19del triple mutant cells in vitro and in vivo.90,92,93 Another study also demonstrated that the combination of brigatinib, osimertinib and the vascular endothelial growth factor (VEGF) inhibitor bevacizumab can be effective against lung adenocarcinomas with the triple L858R-T790M-cis C797S EGFR mutation.94 In the case of uncommon EGFR secondary mutations, such as L718Q and L844V, that are present alongside EGFR-activating mutations, preclinical studies have demonstrated the effectiveness of afatinib and gefitinib in the absence of the T790M mutation.87 Similarly, preclinical studies have shown that the secondary EGFR L718V mutation confers resistance to osimertinib but retains sensitivity to the EGFR inhibitor afatinib.95
Considering that resistance to osimertinib usually involves multiple mechanisms, such as the activation of alternative cellular pathways or aberrant downstream signalling, osimertinib-based combination therapies are being extensively investigated (Table 1). The combination of osimertinib with pemetrexed or cisplatin has been investigated in EGFR-mutant NSCLC preclinical models.96 Furthermore, preliminary results have shown that the addition of platinum-based chemotherapy to osimertinib therapy is well tolerated in EGFR-TKI pre-treated patients.97,98 A Phase 1 study of carboplatin/etoposide plus osimertinib in EGFR-mutated patients with concurrent RB1 and TP53 alterations—in order to prevent SCLC transformation—is currently ongoing (NCT03567642). Taking into account the positive OS results derived from the addition of chemotherapy to first-generation TKIs for the first-line treatment of patients with EGFR-mutated NSCLC,99 randomised trials exploring osimertinib combined with chemotherapy in the same clinical setting are needed.
MET amplification/mutation is a common bypass resistance mechanism to osimertinib, and combining c-Met inhibitors, such as crizotinib, with osimertinib has been found to be effective in osimertinib-resistant EGFR-mutated NSCLC patients harbouring MET amplification.56,57,58 Clinical benefit from the combination of osimertinib and cabozantinib after simultaneous development of secondary MET mutations upon crizotinib treatment has been reported in one patient.100 Preclinical evidence has pointed to the combination of the MEK inhibitor selumetinib with osimertinib as a strategy to prevent osimertinib resistance.62 Moreover, in a recent paper by Suzawa et al.,59 the combination of crizotinib and osimertinib was a successful treatment strategy to tackle acquired MET exon 14 skipping that emerged in one EGFR L858R + T790M-mutated patient. A combination of osimertinib and trastuzumab-emtansine, a conjugate of the monoclonal antibody trastuzumab (Herceptin) and the cytotoxic agent DM1, was reported to overcome osimertinib resistance in T790M-positive EGFR-mutated NSCLC cell lines that gained HER2 amplification.101 Similarly, combining osimertinib with drugs targeting other downstream pathways, such as a BRAF inhibitor64 or the AXL inhibitor cabozantinib,102 constitutes a promising strategy for overcoming osimertinib resistance, but clinical evidence regarding the effectiveness of these approaches is lacking so far. A number of clinical trials are currently underway combining osimertinib with one or more kinase inhibitors. The TATTON study (NCT02143466) is investigating the combination of osimertinib with savolitinib (c-Met inhibitor) or selumetinib (MEK1/2 inhibitor) in patients who progressed following EGFR targeted treatment. In the same trial, the combination of osimertinib and the immune checkpoint inhibitor durvalumab showed encouraging efficacy results, both in TKI-pre-treated and TKI-naïve patients, but enrolment into this arm has been stopped owing to an increase of pulmonary toxicity. Due to the effect of aspirin in reducing AKT phosphorylation, a combination study of osimertinib with aspirin is also ongoing (NCT03532698). Other trials are exploring osimertinib efficacy in combination with bevacizumab (NCT03133546), the Bcl-2 inhibitor navitoclax (NCT02520778) and the mTorc1/2 inhibitor sapanisertinib (NCT02503722). As resistance mechanisms to osimertinib can also involve dysregulation of cell cycle, the combination of osimertinib with a CDK 4/6 inhibitor is being investigated (NCT03455829).
One of the main limitations of targeted therapy such as TKIs is that the tumour response is not durable with inevitable development of drug resistance. However, it has been recognised that acquisition of drug resistance by cancer cells also makes them more sensitive to alternative drugs, a phenomenon referred to as ‘collateral sensitivity’.103 Potentially, this sequential or alternating approach could be used for EGFR-mutant NSCLC. After initial treatment with a given TKI, the drug-resistant cells can be eliminated with a second TKI, following which the population should be sensitive to the first TKI again.103,104 Other authors have suggested that alternating dosing regimens might be superior to the use of intermittent schedules, by selectively targeting and establishing an equilibrium between both drug-sensitive and drug-resistant cancer cell populations.105
Conclusions and future perspectives
The remarkable success of osimertinib for the treatment of patients with advanced EGFR-mutated NSCLC has been mitigated by the development of acquired resistance to this agent. Due to the limited available data about resistance mechanisms to front-line osimertinib, a better molecular characterization of treatment-naïve patients who progressed on osimertinib is eagerly awaited. In the last years, the non-invasive ctDNA genotyping approach has supported tumour re-biopsy in helping to unravel resistance mechanisms to targeted drugs, by providing a wider picture of the heterogeneous molecular events that occur at the time of progression.106 Further implementation of liquid biopsy and integration with RNA sequencing data in monitoring the response to osimertinib and detecting the molecular alterations responsible for treatment failure is warranted. For instance, the mutational status of T790M—whose loss is usually associated with early resistance to osimertinib—could be readily monitored in plasma in order to precede a proven radiological progression of disease. Moreover, the implementation of novel technologies such as CRISPR/Cas9 gene editing will help researchers in creating preclinical models for drug testing and will provide additional insights into the complexity of acquired resistance to osimertinib by high-throughput screening of resistant tumours.107 CRISPR technology is indeed emerging as a versatile tool to analyse gene function and evaluate new therapeutic strategies. CRISPR/Cas‐mediated gene knockout would be expected to be more efficient than RNA-interference‐mediated gene knockdown. For instance, a recent study combined lentiviral transfection with CRISPR/Cas9 techniques to deliver vectors that encode for Cas9 protein and the specific single-guide RNA (sgRNA) to EGFR DNA sequence.108 Similarly, the EGFR‐mutant genes can be repaired or destroyed with virus‐delivered CRISPR/Cas system.109 These ‘molecular surgeries’ on genomic DNA directly target the cause of the resistance in a ‘personalised and possibly permanent manner’. Thus, this novel approach could be combined with traditional chemo- and targeted therapy and should have the potential to significantly improve the survival of patients with EGFR‐mutant NSCLC. More detailed data are expected to emerge from the ELIOS study (NCT03239340), a Phase 2, open-label, single-arm study to assess the efficacy, safety and underlying resistance mechanisms to osimertinib when used as first-line therapy in patients with locally advanced or metastatic EGFR-mutated NSCLC. In this trial, plasma genotyping together with paired tumour biopsy will be analysed by next-generation sequencing. The recently launched ORCHARD Phase 2 trial (NCT03944772) aims to explore treatment options following disease progression on first-line osimertinib in the same clinical setting by investigating the onset of acquired resistance mechanisms.110 In this innovative platform trial, patients will be allocated to a biomarker-matched study treatment—osimertinib plus gefitinib, osimertinib plus savolitinib, osimertinib plus necitumumab, platinum-based doublet plus durvalumab—within each group based on tumour molecular profile.
References
Rosell, R., Moran, T., Queralt, C., Porta, R., Cardenal, F., Camps, C. et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med 361, 958–967 (2009).
Shi, Y., Au, J. S.-K., Thongprasert, S., Srinivasan, S., Tsai, C.-M., Khoa, M. T. et al. A Prospective, Molecular Epidemiology Study of EGFR Mutations in Asian Patients with Advanced Non–Small-Cell Lung Cancer of Adenocarcinoma Histology (PIONEER). J. Thorac. Oncol. 9, 154–162 (2014).
Recondo, G., Facchinetti, F., Olaussen, K. A., Besse, B. & Friboulet, L. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat. Rev. Clin. Oncol. 15, 694–708 (2018).
Lim, S. M., Syn, N. L., Cho, B. C. & Soo, R. A. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Treat. Rev. 65, 1–10 (2018).
Cross, D. A. E., Ashton, S. E., Ghiorghiu, S., Eberlein, C., Nebhan, C. A., Spitzler, P. J. et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Disco. 4, 1046–1061 (2014).
Soria, J.-C., Ohe, Y., Vansteenkiste, J., Reungwetwattana, T., Chewaskulyong, B., Lee, K. H. et al. Osimertinib in untreated EGFR -mutated advanced non–small-cell lung cancer. N. Engl. J. Med 378, 113–125 (2018).
Jänne, P. A., Yang, J. C.-H., Kim, D.-W., Planchard, D., Ohe, Y., Ramalingam, S. S. et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N. Engl. J. Med 372, 1689–1699 (2015).
Yang, J. C.-H., Ahn, M.-J., Kim, D.-W., Ramalingam, S. S., Sequist, L. V., Su, W.-C. et al. Osimertinib in pretreated T790M-positive advanced non–small-cell lung cancer: AURA study phase II extension component. J. Clin. Oncol. 35, 1288–1296 (2017).
Goss, G., Tsai, C. M., Shepherd, F. A., Bazhenova, L., Lee, J. S., Chang, G. C. et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 17, 1643–1652 (2016).
Mok, T. S., Wu, Y.-L., Ahn, M.-J., Garassino, M. C., Kim, H. R., Ramalingam, S. S. et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med 376, 629–640 (2017).
Ahn, M.-J., Tsai, C.-M., Shepherd, F. A., Bazhenova, L., Sequist, L. V., Hida, T. et al. Osimertinib in patients with T790M mutation‐positive, advanced non–small cell lung cancer: long‐term follow‐up from a pooled analysis of 2 phase 2 studies. Cancer 125, 892–901 (2019).
Ramalingam, S. S., Yang, J. C. H., Lee, C. K., Kurata, T., Kim, D.-W., John, T. et al. Osimertinib as first-line treatment of egfr mutation–positive advanced non–small-cell lung cancer. J. Clin. Oncol. 36, 841–849 (2018).
Planchard, D., Popat, S., Kerr, K., Novello, S., Smit, E. F., Faivre-Finn, C. et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29, iv192–iv237 (2018).
Heon, S., Yeap, B. Y., Britt, G. J., Costa, D. B., Rabin, M. S., Jackman, D. M. et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin. Cancer Res 16, 5873–5882 (2010).
Ballard, P., Yates, J. W. T., Yang, Z., Kim, D.-W., Yang, J. C.-H., Cantarini, M. et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin. Cancer Res 22, 5130–5140 (2016).
Leonetti, A., Facchinetti, F. & Tiseo, M. Upfront osimertinib in EGFR-mutated non-small cell lung cancer: is brain still a sanctuary? Ann. Transl. Med 6, S110 (2018).
Wu, Y.-L., Ahn, M.-J., Garassino, M. C., Han, J.-Y., Katakami, N., Kim, H. R. et al. CNS efficacy of osimertinib in patients with T790M-Positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J. Clin. Oncol. 36, 2702–2709 (2018).
Reungwetwattana, T., Nakagawa, K., Cho, B. C., Cobo, M., Cho, E. K., Bertolini, A. et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR -mutated advanced non–small-cell lung cancer. J. Clin. Oncol. 36, 3290–3297 (2018).
Yang, J. C.-H., Cho, B. C., Kim, D.-W., Kim, S.-W., Lee, J.-S., Su, W.-C. et al. Osimertinib for patients (pts) with leptomeningeal metastases (LM) from EGFR-mutant non-small cell lung cancer (NSCLC): Updated results from the BLOOM study. J. Clin. Oncol. 35, 2020–2020 (2018).
Papadimitrakopoulou V. A., Wu Y.-L., Han J.-Y., Ahn M.-J., Ramalingam S. S., John T. et al. LBA51Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. Ann. Oncol. 29, https://doi.org/10.1093/annonc/mdy424.064 (2018).
Oxnard, G. R., Hu, Y., Mileham, K. F., Husain, H., Costa, D. B., Tracy, P. et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M–positive lung cancer and acquired resistance to osimertinib. JAMA Oncol. 4, 1527–1534 (2018).
Lin, C. C., Shih, J. Y., Yu, C. J., Ho, C. C., Liao, W. Y., Lee, J. H. et al. Outcomes in patients with non-small-cell lung cancer and acquired Thr790Met mutation treated with osimertinib: a genomic study. Lancet Respir. Med 6, 107–116 (2018).
Le, X., Puri, S., Negrao, M. V., Nilsson, M. B., Robichaux, J., Boyle, T. et al. Landscape of EGFR-dependent and -independent resistance mechanisms to osimertinib and continuation therapy beyond progression in EGFR-mutant NSCLC. Clin. Cancer Res 24, 6195–6203 (2018).
Zhao, S., Li, X., Zhao, C., Jiang, T., Jia, Y., Shi, J. et al. Loss of T790M mutation is associated with early progression to osimertinib in Chinese patients with advanced NSCLC who are harboring EGFR T790M. Lung Cancer 128, 33–39 (2019).
Ramalingam S. S., Cheng Y., Zhou C., Ohe Y., Imamura F., Cho B. C. et al. LBA50Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Ann. Oncol. 29 https://doi.org/10.1093/annonc/mdy424.063 (2018).
Yang, Z., Yang, N., Ou, Q., Xiang, Y., Jiang, T., Wu, X. et al. Investigating novel resistance mechanisms to third-generation egfr tyrosine kinase inhibitor osimertinib in non–small cell lung cancer patients. Clin. Cancer Res 24, 3097–3107 (2018).
Jiang, T., Su, C., Ren, S., Cappuzzo, F., Rocco, G., Palmer, J. D. et al. A consensus on the role of osimertinib in non-small cell lung cancer from the AME Lung Cancer Collaborative Group. J. Thorac. Dis. 10, 3909–3921 (2018).
Niederst, M. J., Hu, H., Mulvey, H. E., Lockerman, E. L., Garcia, A. R., Piotrowska, Z. et al. The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin. Cancer Res 21, 3924–3933 (2015).
Wu, Y. L., Hodge, R., Papadimitrakopoulou, V., He, Y., Mok, T., Delmonte, A. et al. MA08.03 osimertinib vs platinum-pemetrexed for T790M-mutation positive advanced NSCLC (AURA3): plasma ctDNA analysis. J. Thorac. Oncol. 12, S386 (2017).
Wang, Z., Yang, J. J., Huang, J., Ye, J. Y., Zhang, X. C., Tu, H. Y. et al. Lung adenocarcinoma harboring EGFR T790M and in trans C797S responds to combination therapy of first- and third-generation EGFR TKIs and shifts allelic configuration at resistance. J. Thorac. Oncol. 12, 1723–1727 (2017).
Arulananda, S., Do, H., Musafer, A., Mitchell, P., Dobrovic, A., John, T. Combination osimertinib and gefitinib in C797S and T790M EGFR-mutated non–small cell lung cancer. J. Thorac. Oncol. 12, 1728–1732 (2017).
Menon, R., Müller, J., Schneider, P., Lakis, S., Thress, K., Wolf, J. et al. A novel EGFR C797 variant detected in a pleural biopsy specimen from an osimertinib-treated patient using a comprehensive hybrid capture–based next-generation sequencing assay. J. Thorac. Oncol. 11, e105–e107 (2016).
Zhang, Q., Zhang, X.-C., Yang, J.-J., Yang, Z.-F., Bai, Y., Su, J. et al. EGFR L792H and G796R: two novel mutations mediating resistance to the third-generation EGFR Tyrosine kinase inhibitor osimertinib. J. Thorac. Oncol. 13, 1415–1421 (2018).
Ou, S.-H. I., Cui, J., Schrock, A. B., Goldberg, M. E., Zhu, V. W., Albacker, L. et al. Emergence of novel and dominant acquired EGFR solvent-front mutations at Gly796 (G796S/R) together with C797S/R and L792F/H mutations in one EGFR (L858R/T790M) NSCLC patient who progressed on osimertinib. Lung Cancer 108, 228–231 (2017).
Zheng, D., Hu, M., Bai, Y., Zhu, X., Lu, X., Wu, C. et al. EGFR G796D mutation mediates resistance to osimertinib. Oncotarget 8, 49671–49679 (2017).
Bersanelli, M., Minari, R., Bordi, P., Gnetti, L., Bozzetti, C., Squadrilli, A. et al. L718Q mutation as new mechanism of acquired resistance to AZD9291 in EGFR -mutated NSCLC. J. Thorac. Oncol. 11, e121–e123 (2016).
Ercan, D., Choi, H. G., Yun, C.-H., Capelletti, M., Xie, T., Eck, M. J. et al. EGFR Mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin. Cancer Res 21, 3913–3923 (2015).
Zhang, Y., He, B., Zhou, D., Li, M. & Hu, C. Newly emergent acquired EGFR exon 18 G724S mutation after resistance of a T790M specific EGFR inhibitor osimertinib in non-small-cell lung cancer: a case report. Onco Targets Ther. 12, 51–56 (2018).
Fassunke, J., Müller, F., Keul, M., Michels, S., Dammert, M. A., Schmitt, A. et al. Overcoming EGFRG724S-mediated osimertinib resistance through unique binding characteristics of second-generation EGFR inhibitors. Nat. Commun. 9, 4655 (2018).
Peled, N., Roisman, L. C., Miron, B., Pfeffer, R., Lanman, R. B., Ilouze, M. et al. Subclonal therapy by two EGFR TKIs guided by sequential plasma cell-free DNA in EGFR -mutated lung cancer. J. Thorac. Oncol. 12, e81–e84 (2017).
Oztan, A., Fischer, S., Schrock, A. B., Erlich, R. L., Lovly, C. M., Stephens, P. J. et al. Emergence of EGFR G724S mutation in EGFR-mutant lung adenocarcinoma post progression on osimertinib. Lung Cancer 111, 84–87 (2017).
Schoenfeld, A. J., Chan, J. M., Rizvi, H., Rekhtman, N., Daneshbod, Y., Kubota, D. et al. Tissue-based molecular and histological landscape of acquired resistance to osimertinib given initially or at relapse in patients with EGFR-mutant lung cancers. J. Clin. Oncol. 37, 9028 (2019).
Brown, B. P., Zhang, Y.-K., Westover, D., Yan, Y., Qiao, H., Huang, V. et al. On-target resistance to the mutant-selective EGFR Inhibitor osimertinib can develop in an allele-specific manner dependent on the original EGFR-activating mutation. Clin. Cancer Res 25, 3341–3351 (2019).
Leventakos, K., Kipp, B. R., Rumilla, K. M., Winters, J. L., Yi, E. S. & Mansfield, A. S. S768I mutation in EGFR in patients with lung cancer. J. Thorac. Oncol. 11, 1798–1801 (2016).
Nukaga, S., Yasuda, H., Tsuchihara, K., Hamamoto, J., Masuzawa, K., Kawada, I. et al. Amplification of EGFR wild-type alleles in non–small cell lung cancer cells confers acquired resistance to mutation-selective EGFR tyrosine kinase inhibitors. Cancer Res 77, 2078–2089 (2017).
Knebel, F. H., Bettoni, F., Shimada, A. K., Cruz, M., Alessi, J. V., Negrão, M. V. et al. Sequential liquid biopsies reveal dynamic alterations of EGFR driver mutations and indicate EGFR amplification as a new mechanism of resistance to osimertinib in NSCLC. Lung Cancer 108, 238–241 (2017).
Shi, Y., Xing, P., Han, X., Wang, S., Liu, Y., Liu, P. et al. P1.13-18 exploring the resistance mechanism of osimertinib and monitoring the treatment response using plasma ctDNA in Chinese NSCLC patients. J. Thorac. Oncol. 13, S589 (2018).
Kim, T. M., Song, A., Kim, D.-W., Kim, S., Ahn, Y.-O., Keam, B. et al. Mechanisms of acquired resistance to AZD9291. J. Thorac. Oncol. 10, 1736–1744 (2015).
Yu, H. A., Arcila, M. E., Rekhtman, N., Sima, C. S., Zakowski, M. F., Pao, W. et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res 19, 2240–2247 (2013).
Chabon, J. J., Simmons, A. D., Lovejoy, A. F., Esfahani, M. S., Newman, A. M., Haringsma, H. J. et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat. Commun. 7, 11815 (2016).
Shi, P., Oh, Y.-T., Zhang, G., Yao, W., Yue, P., Li, Y. et al. Met gene amplification and protein hyperactivation is a mechanism of resistance to both first and third generation EGFR inhibitors in lung cancer treatment. Cancer Lett. 380, 494–504 (2016).
Martinez-Marti, A., Felip, E., Matito, J., Mereu, E., Navarro, A., Cedrés, S. et al. Dual MET and ERBB inhibition overcomes intratumor plasticity in osimertinib-resistant-advanced non-small-cell lung cancer (NSCLC). Ann. Oncol. 28, 2451–2457 (2017).
O’Kane, G. M., Barnes, T. A. & Leighl, N. B. Resistance to epidermal growth factor receptor tyrosine kinase inhibitors, T790M, and clinical trials. Curr. Oncol. 25, S28–S37 (2018).
Ortiz-Cuaran, S., Scheffler, M., Plenker, D., Dahmen, L., Scheel, A. H., Fernandez-Cuesta, L. et al. Heterogeneous Mechanisms of Primary and Acquired Resistance to Third-Generation EGFR Inhibitors. Clin. Cancer Res 22, 4837–4847 (2016).
Xu C., Wang W., Zhu Y., Yu Z., Zhang H., Wang H. et al. 114OPotential resistance mechanisms using next generation sequencing from Chinese EGFR T790M+ non-small cell lung cancer patients with primary resistance to osimertinib: a multicenter study. Ann. Oncol. 30, https://doi.org/10.1093/annonc/mdz063.012 (2019).
York, E. R., Varella-Garcia, M., Bang, T. J., Aisner, D. L. & Camidge, D. R. Tolerable and Effective Combination of Full-Dose Crizotinib and Osimertinib Targeting MET Amplification Sequentially Emerging after T790M Positivity in EGFR- Mutant Non–Small Cell Lung Cancer. J. Thorac. Oncol. 12, e85–e88 (2017).
Deng, L., Kiedrowski, L. A., Ravera, E., Cheng, H. & Halmos, B. Response to dual crizotinib and osimertinib treatment in a lung cancer patient with MET amplification detected by liquid biopsy who acquired secondary resistance to EGFR tyrosine kinase inhibition. J. Thorac. Oncol. 13, e169–e172 (2018).
Zhu, V. W., Schrock, A. B., Ali, S. M., Ou & S-HI. Differential response to a combination of full-dose osimertinib and crizotinib in a patient with EGFR-mutant non-small cell lung cancer and emergent MET amplification. Lung Cancer Targets Ther. 10, 21–26 (2019).
Suzawa, K., Offin, M., Schoenfeld, A. J., Plodkowski, A. J., Odintsov, I., Lu, D. et al. Acquired MET exon 14 alteration drives secondary resistance to epidermal growth factor receptor tyrosine kinase inhibitor in EGFR -mutated lung cancer. JCO Precis Oncol. 3, 1–8 (2019).
Planchard, D., Loriot, Y., André, F., Gobert, A., Auger, N., Lacroix, L. et al. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann. Oncol. 26, 2073–2078 (2015).
Takezawa, K., Pirazzoli, V., Arcila, M. E., Nebhan, C. A., Song, X., de Stanchina, E. et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Disco. 2, 922–933 (2012).
Eberlein, C. A., Stetson, D., Markovets, A. A., Al-Kadhimi, K. J., Lai, Z., Fisher, P. R. et al. Acquired resistance to the mutant-selective EGFR inhibitor AZD9291 is associated with increased dependence on RAS signaling in preclinical models. Cancer Res 75, 2489–2500 (2015).
Hong M. H., Kim M. H., Kim S.-Y., Heo S. G., Kang H.-N., Park C.-W. et al. 1429PMolecular landscape of osimertinib resistance revealed by targeted panel sequencing and patient-derived cancer models in non-small cell lung cancer patients. Ann. Oncol. 29, https://doi.org/10.1093/annonc/mdy292.051 (2018).
Ho, C.-C., Liao, W.-Y., Lin, C.-A., Shih, J.-Y., Yu, C.-J. & Chih-Hsin Yang, J. Acquired BRAF V600E mutation as resistant mechanism after treatment with osimertinib. J. Thorac. Oncol. 12, 567–572 (2017).
Oxnard, G., Thress, K., Paweletz, D., Stetson, D., Dougherty, B., Lai, Z. et al. Mechanisms of acquired resistance to AZD9291 in EGFRT790M positive lung cancer. J. Thorac. Oncol. 10, S173–S260 (2015).
Minari, R., Bordi, P., La Monica, S., Squadrilli, A., Leonetti, A., Bottarelli, L. et al. Concurrent acquired BRAF V600E mutation and MET amplification as resistance mechanism of first-line osimertinib treatment in a patient with EGFR-mutated NSCLC. J. Thorac. Oncol. 13, e89–e91 (2018).
Barnes, T. A., O’Kane, G. M., Vincent, M. D. & Leighl, N. B. Third-generation tyrosine kinase inhibitors targeting epidermal growth factor receptor mutations in non-small cell lung cancer. Front Oncol. 7, 113 (2017).
Eng, J., Woo, K. M., Sima, C. S., Plodkowski, A., Hellmann, M. D., Chaft, J. E. et al. Impact of concurrent PIK3CA mutations on response to EGFR tyrosine kinase inhibition in EGFR-mutant lung cancers and on prognosis in oncogene-driven lung adenocarcinomas. J. Thorac. Oncol. 10, 1713–1719 (2015).
Gupta, S., Ramjaun, A. R., Haiko, P., Wang, Y., Warne, P. H., Nicke, B. et al. Binding of Ras to phosphoinositide 3-Kinase p110α is required for Ras- driven tumorigenesis in mice. Cell 129, 957–968 (2007).
Blakely, C. M., Watkins, T. B. K., Wu, W., Gini, B., Chabon, J. J., McCoach, C. E. et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat. Genet 49, 1693–1704 (2017).
Piotrowska, Z., Isozaki, H., Lennerz, J. K., Gainor, J. F., Lennes, I. T., Zhu, V. W. et al. Landscape of acquired resistance to osimertinib in EGFR -mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired RET fusion. Cancer Disco. 8, 1529–1539 (2018).
Zeng, L., Yang, N. & Zhang, Y. GOPC - ROS1 rearrangement as an acquired resistance mechanism to osimertinib and responding to crizotinib combined treatments in lung adenocarcinoma. J. Thorac. Oncol. 13, e114–e116 (2018).
Schrock, A. B., Zhu, V. W., Hsieh, W.-S., Madison, R., Creelan, B., Silberberg, J. et al. Receptor tyrosine kinase fusions and BRAF kinase fusions are rare but actionable resistance mechanisms to EGFR tyrosine kinase inhibitors. J. Thorac. Oncol. 13, 1312–1323 (2018).
Offin, M., Somwar, R., Rekhtman, N., Benayed, R., Chang, J. C., Plodkowski, A. et al. Acquired ALK and RET gene fusions as mechanisms of resistance to osimertinib in EGFR -mutant lung cancers. JCO Precis Oncol. 2, 1–12 (2018).
Minari, R., Bordi, P., Del Re, M., Facchinetti, F., Mazzoni, F., Barbieri, F. et al. Primary resistance to osimertinib due to SCLC transformation: Issue of T790M determination on liquid re-biopsy. Lung Cancer 115, 21–27 (2018).
Li, L., Wang, H., Li, C., Wang, Z., Zhang, P. & Yan, X. Transformation to small-cell carcinoma as an acquired resistance mechanism to AZD9291: a case report. Oncotarget 8, 18609–18614 (2017).
Ham, J. S., Kim, S., Kim, H. K., Byeon, S., Sun, J.-M., Lee, S. et al. Two cases of small cell lung cancer transformation from EGFR mutant adenocarcinoma during AZD9291 treatment. J. Thorac. Oncol. 11, e1–e4 (2016).
Taniguchi, Y., Horiuchi, H., Morikawa, T. & Usui, K. Small-cell carcinoma transformation of pulmonary adenocarcinoma after osimertinib treatment: a case report. Case Rep. Oncol. 11, 323–329 (2018).
Sequist, L. V., Waltman, B. A., Dias-Santagata, D., Digumarthy, S., Turke, A. B., Fidias, P. et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med 3, 75ra26 (2011).
Lee, J.-K., Lee, J., Kim, S., Kim, S., Youk, J., Park, S. et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J. Clin. Oncol. 35, 3065–3074 (2017).
Niederst, M. J., Sequist, L. V., Poirier, J. T., Mermel, C. H., Lockerman, E. L., Garcia, A. R. et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat. Commun. 6, 6377 (2015).
Weng, C.-H., Chen, L.-Y., Lin, Y.-C., Shih, J.-Y., Lin, Y.-C., Tseng, R.-Y. et al. Epithelial-mesenchymal transition (EMT) beyond EGFR mutations per se is a common mechanism for acquired resistance to EGFR TKI. Oncogene 38, 455–468 (2019).
Ichihara, E., Westover, D., Meador, C. B., Yan, Y., Bauer, J. A., Lu, P. et al. SFK/FAK signaling attenuates osimertinib efficacy in both drug-sensitive and drug-resistant models of EGFR-mutant lung cancer. Cancer Res 77, 2990–3000 (2017).
Yochum, Z. A., Cades, J., Wang, H., Chatterjee, S., Simons, B. W., O’Brien, J. P. et al. Targeting the EMT transcription factor TWIST1 overcomes resistance to EGFR inhibitors in EGFR-mutant non-small-cell lung cancer. Oncogene 38, 656–670 (2019).
Taniguchi, H., Yamada, T., Wang, R., Tanimura, K., Adachi, Y., Nishiyama, A. et al. AXL confers intrinsic resistance to osimertinib and advances the emergence of tolerant cells. Nat. Commun. 10, 259 (2019).
Ricordel, C., Friboulet, L., Facchinetti, F. & Soria, J.-C. Molecular mechanisms of acquired resistance to third-generation EGFR-TKIs in EGFR T790M-mutant lung cancer. Ann. Oncol. 29, i28–i37 (2018).
Pakkala, S. & Ramalingam, S. S. Personalized therapy for lung cancer: striking a moving target. JCI Insight 3, 120858 (2018).
Engel, J., Richters, A., Getlik, M., Tomassi, S., Keul, M., Termathe, M. et al. Targeting drug resistance in EGFR with covalent inhibitors: a structure-based design approach. J. Med Chem. 58, 6844–6863 (2015).
Wang, S., Song, Y. & Liu, D. EAI045: the fourth-generation EGFR inhibitor overcoming T790M and C797S resistance. Cancer Lett. 385, 51–54 (2017).
Noda-Narita, S. & Kanda, S. Overcoming resistance to third-generation epidermal growth factor receptor tyrosine kinase inhibitor in non-small cell lung cancer. Transl. Cancer Res 6, S1187–S1190 (2017).
To, C., Jang, J., Chen, T., Park, E., Mushajiang, M., De Clercq, D. J. H. et al. Single and dual targeting of mutant EGFR with an allosteric inhibitor. Cancer Disco. 9, 926–943 (2019).
Uchibori, K., Inase, N., Araki, M., Kamada, M., Sato, S., Okuno, Y. et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat. Commun. 8, 14768 (2017).
Wang, X., Zhou, L., Yin, J. C., Wu, X., Shao, Y. W. & Gao, B. Lung Adenocarcinoma harboring EGFR 19del/C797S/T790M triple mutations responds to brigatinib and Anti-EGFR antibody combination therapy. J. Thorac. Oncol. 14, e85–e88 (2019).
Zhao, J., Zou, M., Lv, J., Han, Y., Wang, G. & Wang, G. Effective treatment of pulmonary adenocarcinoma harboring triple EGFR mutations of L858R, T790M, and cis-C797S by osimertinib, bevacizumab, and brigatinib combination therapy: a case report. Onco Targets Ther. 11, 5545–5550 (2018).
Liu, Y., Li, Y., Ou, Q., Wu, X., Wang, X., Shao, Y. W. et al. Acquired EGFR L718V mutation mediates resistance to osimertinib in non-small cell lung cancer but retains sensitivity to afatinib. Lung Cancer 118, 1–5 (2018).
La Monica, S., Minari, R., Cretella, D., Flammini, L., Fumarola, C., Bonelli, M. et al. Third generation EGFR inhibitor osimertinib combined with pemetrexed or cisplatin exerts long-lasting anti-tumor effect in EGFR-mutated pre-clinical models of NSCLC. J. Exp. Clin. Cancer Res 38, 222 (2019).
Piotrowska, Z., Liu, S. V., Muzikansky, A., Marcoux, N., Banwait, M., Stevens, S. et al. Safety of osimertinib plus chemotherapy in EGFR-mutant NSCLC. J. Clin. Oncol. 36, e21231–e21231 (2018).
Okada, M., Tanaka, K., Asahina, H., Harada, T., Hamai, K., Watanabe, K. et al. Safety analysis of an open label, randomized phase 2 study of osimertinib alone versus osimertinib plus carboplatin-pemetrexed for patients with non–small cell lung cancer (NSCLC) that progressed during prior epidermal growth factor receptor (EGFR) tyrosi. J. Clin. Oncol. 36, e21073–e21073 (2018).
Nakamura, A., Inoue, A., Morita, S., Hosomi, Y., Kato, T., Fukuhara, T. et al. Phase III study comparing gefitinib monotherapy (G) to combination therapy with gefitinib, carboplatin, and pemetrexed (GCP) for untreated patients (pts) with advanced non-small cell lung cancer (NSCLC) with EGFR mutations (NEJ009). J. Clin. Oncol. 36, 9005–9005 (2018).
Kang, J., Chen, H.-J., Wang, Z., Liu, J., Li, B., Zhang, T. et al. Osimertinib and cabozantinib combinatorial therapy in an EGFR -mutant lung adenocarcinoma patient with multiple MET secondary-site mutations after resistance to crizotinib. J. Thorac. Oncol. 13, e49–e53 (2018).
La Monica, S., Cretella, D., Bonelli, M., Fumarola, C., Cavazzoni, A., Digiacomo, G. et al. Trastuzumab emtansine delays and overcomes resistance to the third-generation EGFR-TKI osimertinib in NSCLC EGFR mutated cell lines. J. Exp. Clin. Cancer Res 36, 174 (2017).
Tian, Y., Zhang, Z., Miao, L., Yang, Z., Yang, J, Wang, Y. et al. Anexelekto (AXL) increases resistance to EGFR-TKI and activation of AKT and ERK1/2 in non-small cell lung cancer cells. Oncol Res 24, 295–303 (2016).
Wang, L. & Bernards, R. Taking advantage of drug resistance, a new approach in the war on cancer. Front Med 12, 490–495 (2018).
Amirouchene-Angelozzi, N., Swanton, C. & Bardelli, A. Tumor evolution as a therapeutic target. Cancer Discov. 7, 805–817 (2017).
Leite de Oliveira, R., Wang, L. & Bernards, R. With great power comes great vulnerability. Mol. Cell Oncol. 5, e1509488 (2018).
Oxnard, G. R., Thress, K. S., Alden, R. S., Lawrance, R., Paweletz, C. P., Cantarini, M. et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non–small-cell lung cancer. J. Clin. Oncol. 34, 3375–3382 (2016).
Murtuza, A., Bulbul, A., Shen, J. P., Keshavarzian, P., Woodward, B. D., Lopez-Diaz, F. J. et al. Novel third-generation EGFR tyrosine kinase inhibitors and strategies to overcome therapeutic resistance in lung cancer. Cancer Res 79, 689–698 (2019).
Huang, L.-C., Tam, K.-W., Liu, W.-N., Lin, C.-Y., Hsu, K.-W., Hsieh, W.-S. et al. CRISPR/Cas9 genome editing of epidermal growth factor receptor sufficiently abolished oncogenicity in anaplastic thyroid cancer. Dis. Markers 2018, 1–14 (2018).
Tang, H. & Shrager, J. B. CRISPR/Cas-mediated genome editing to treat EGFR-mutant lung cancer: a personalized molecular surgical therapy. EMBO Mol. Med 8, 83–85 (2016).
New data on mechanisms of acquired resistance after 1st-line Tagrisso in NSCLC support initiation of ORCHARD trial to explore post-progression treatment options. https://www.astrazeneca.com/media-centre/press-releases/2018/new-data-on-mechanisms-of-acquired-resistance-after-1st-line-tagrisso-in-nsclc-support-initiation-of-orchard-trial-to-explore-post-progression-treatment-options-19102018.html (2019).
Author information
Authors and Affiliations
Contributions
A.L., S.S., M.T. and E.G. designed the review; A.L., S.S., R.M., P.P., E.G. and M.T. contributed to the writing of the paper.
Corresponding author
Ethics declarations
Competing interests
M.T.: advisory boards and speakers’ fee for Astra-Zeneca. The remaining authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Funding
Grant by AIRC (Italian Association for Cancer Research) to M.T. (grant IG2017-20074), grant by Fondazione CARIPLO-Regione Lombardia to P.P. (grant 2016-1019), grants by Italian Association for Cancer Research, AIRC/Start-Up grant, and by Fondazione Pisana per la Scienza, to E.G.
Consent to publish
Not applicable.
Data availability
Not applicable.
Additional information
Note: This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leonetti, A., Sharma, S., Minari, R. et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 121, 725–737 (2019). https://doi.org/10.1038/s41416-019-0573-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-019-0573-8
This article is cited by
-
Impact of medical insurance access negotiation on the utilization of innovative anticancer drugs in China: an interrupted time series analysis
BMC Health Services Research (2024)
-
The difference between dacomitinib and afatinib in effectiveness and safety in first-line treatment of patients with advanced EGFR-mutant non-small cell lung cancer: a real-world observational study
BMC Cancer (2024)
-
Chemotherapy versus personalized therapy for EGFR mutant lung adenocarcinoma resistance to EGFR-tyrosine kinase inhibitors: a retrospective dual-center study
BMC Pulmonary Medicine (2024)
-
Unraveling EGFR-TKI resistance in lung cancer with high PD-L1 or TMB in EGFR-sensitive mutations
Respiratory Research (2024)
-
Single cell lineage tracing reveals clonal dynamics of anti-EGFR therapy resistance in triple negative breast cancer
Genome Medicine (2024)