Abstract
Background
Consolidation treatment with an anti-PD-L1 antibody, durvalumab, following concurrent chemo-radiotherapy (cCRT) has become a new standard of care for locally advanced non-small cell lung cancer (NSCLC). The rationale of PD-L1 blockade after cCRT is based on preclinical evidence suggesting that chemotherapy and radiotherapy up-regulate tumoural PD-L1 expression, which has not been shown in clinical studies.
Methods
To examine alteration in tumoural PD-L1 expression (tumour proportion score, TPS) and density of stromal CD8-positive tumour-infiltrating lymphocytes (CD8 + TILs) after cCRT, paired NSCLC samples obtained before and after cCRT were reviewed in comparison with those obtained before and after drug therapy.
Results
PD-L1 expression was significantly up-regulated after cCRT (median TPS, 1.0 at baseline versus 48.0 after cCRT; P < 0.001), but not after drug therapy. There was no significant correlation between baseline TPS and post-cCRT TPS. CD8 + TIL density was significantly increased after cCRT (median, 10.6 versus 39.1; P < 0.001), and higher post-cCRT CD8 + TIL density was associated with a higher pathologic response and with a favourable survival (P = 0.019).
Conclusion
Tumoural PD-L1 expression was up-regulated after cCRT, which provides pathologic rationale for PD-L1 blockade following cCRT to improve prognosis. Stromal CD8 + TIL density was also increased after cCRT, and higher post-cCRT CD8 + TIL density was a favourable prognostic indicator.
Similar content being viewed by others
Background
Non-small cell lung cancer (NSCLC) accounts for ~85% of lung cancer, which is the leading cause of cancer deaths worldwide.1 More than 30% of NSCLC patients present with locally advanced and unresectable disease, and concurrent chemo-radiotherapy (cCRT) has been employed as a standard treatment of care for such patients. However, the prognosis achieved with cCRT remains un-satisfactory with the 5-year overall survival (OS) rate of 15%.2 Recently, a randomised controlled trial demonstrated that the consolidation treatment with durvalumab, an antibody against programmed death ligand-1 (PD-L1), provided a significant survival benefit among patients who did not have disease progression after cCRT.3,4 Based on the results, durvalumab treatment following cCRT has become a new standard of care for locally advanced and unresectable NSCLC patients with good performance status (PS).5
The rationale of addition of PD-L1 blockade following cCRT is based on preclinical evidence suggesting that chemotherapy6 and radiotherapy7,8 up-regulate PD-L1 expression on tumour cells. Up-regulation of tumoural PD-L1 leads to acquired resistance to radiotherapy, which may be overcome by PD-L1 blockade.7,8 However, there has been reported no clinical evidence showing up-regulation of tumoural PD-L1 expression after cCRT. Only one clinical study to examine alteration in PD-L1 expression by using paired NSCLC samples obtained before and after cCRT showed a significant decrease in PD-L1 expression after cCRT,9 which is contrary to preclinical results.7,8 In the present study, to assess the impact of cCRT on tumour immune microenvironment in NSCLC, we examined alteration in tumoural PD-L1 expression and in density of stromal CD8-positive tumour-infiltrating lymphocytes (CD8 + TILs) in paired histologic samples that had been obtained before and after cCRT in comparison with that in paired specimens obtained before and after drug therapy.
Methods
Patients
NSCLC patients who received pre-operative treatment followed by surgery from January 2008 through December 2017 at our institute were retrospectively reviewed. Patients were eligible when paired tumour specimens obtained before and after preoperative treatment were available for pathological evaluation and for immunohistochemistry (IHC) to examine tumoural PD-L1 expression and stromal CD8 + TILs. No patients received radiotherapy alone. Seven patients who achieved pathologic complete response with preoperative treatment were excluded, because tumoural PD-L1 expression could not be evaluable. A total of 23 patients who underwent cCRT were included and were reviewed in comparison with 18 patients who underwent drug therapy alone as preoperative treatment (Supplementary Fig. 1).
Clinical stage (c-stage) was determined according to the current tumour, node, metastases (TNM) classification.10 Pathological response to preoperative treatment was evaluated according to the ‘General rule for clinical and pathological record of lung cancer (7th edition) by the Japan Lung Cancer Society’ as follows: therapeutic effect 0, no therapeutic effect; 1, residual viable cancer cells detected in ‘≥1/3’ of resected tumour; 2, residual viable cancer cells detected in ‘<1/3’ of resected tumour; 3, no residual viable cancer cells.11
Evaluation tumour immune microenvironment
PD-L1 expression on tumour cells and density of stromal CD8 + TILs were evaluated with IHC.9,12,13,14 Serial 4-µm sections were cut from each formalin-fixed and paraffin-embedded tumour specimen. For evaluation of PD-L1 expression, sections were heated in 1 mmol/L ethylenediaminetetraacetic acid (pH 8.0) at 98°C for 15 min for antigen retrieval, and were incubated in 3% hydrogen peroxide for 10 min to inactivate endogenous peroxidase. After blocking with Protein Block Serum-Free (Agilent Technologies, Carpinteria, CA) for 30 min, sections were incubated with a rabbit anti-PD-L1 monoclonal antibody (clone E1L3N,12,13 Cell Signaling Technology Japan, Tokyo, Japan) diluted at 1:200 for 1 h at room temperature. Sections were then washed and incubated with SignalStain Boost IHC Detection Reagent HRP Rabbit (Cell Signaling Technology Japan) for 30 min. Thereafter, they were visualised with DAB + Liquid (Agilent Technologies) and counterstained with haematoxylin. Paraffin-embedded cell pellets (KARPAS-299 as PD-L1-positive cells and PC-3 as PD-L1 negative cells, Cell Signaling Technology Japan) served as controls for PD-L1 immuno-staining. For evaluation of CD8-positive lymphocytes, slide was stained with an anti-CD8 antibody (clone C8/144B; Aglient) using the DAKO Autostainer Link 48 (Agilent Technologies) according to the manufacturer’s protocol.
Each slide was independently evaluated by two of the investigators (K.Y. and F.T.) without the knowledge of any clinical data. The percentage of tumour cells with membrane staining for PD-L1 was recorded and represented as tumour proportion score (TPS). A minimum of 100 tumour cells were evaluated to calculate TPS. The percentage of CD8-positive lymphocytes among total nucleated cells in the stromal compartments were defined as the CD8 + TIL density.9,14 When a discrepancy was found between the two investigators, the slide was reviewed via their simultaneous examination using a double-headed microscope to achieve a consensus.
Statistical analysis
Proportions of categorical data were compared by the chi-square test or the Fisher’s exact test, as appropriate. Continuous data were compared with a non-parametric test (Wilcoxon signed rank test for paired data or Mann–Whitney U-test for un-paired data). Spearman’s rank correlation coefficients (two-sided) were used to evaluate correlations between two parameters. Receiver operating characteristic (ROC) curve analyses were performed to determine the optimal cut-off value of TPS for PD-L1 expression and CD8 + TIL density.
A telephone inquiry was made if the patient did not come to our clinic for a routine follow-up. The Kaplan–Meier method was used to estimate probability of recurrence-free survival (RFS) and OS, and survival differences were analysed by the log-rank test. The hazard ratio (HR) and 95% confidence interval (CI) were calculated for each variable.
Differences were considered to be statistically significant for P-values < 0.05. All statistical analyses were performed with the SPSS version 21 software (IBM, Armonk, NY).
Results
Patient characteristics
Among 23 patients who received cCRT, the majority of patients had c-stage III disease. All patients received platinum-based chemotherapy in combination with concurrent radiotherapy with the median dose of 60 Gy followed by complete resection. Seventeen (73.9%) of 23 patients achieved therapeutic effect 2 with cCRT. In the drug-therapy group, only five (27.8%) of 18 patients achieved therapeutic effect 2 with pre-operative treatment (Table 1).
Alteration in PD-L1 expression on tumour cells
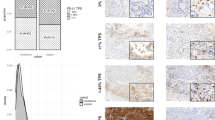
After cCRT, 21 patients (91.3%) showed an increase in TPS, and the other two patients showed a decrease. After drug therapy, 10 patients showed an increase in TPS, whereas the other eight patients showed a decrease. TPS was significantly increased after cCRT (median TPS, 1.0 at the baseline versus 48.0 after cCRT; P < 0.001) but was not after drug therapy (Fig. 1). The post-treatment TPS was significantly higher in the cCRT group than in the drug-therapy group (median TPS, 48.0 and 7.5, respectively; P < 0.001), whereas baseline TPS was similar (median TPS, 1.0 in both group; P = 0.959). There was no significant correlation between baseline TPS of and post-treatment TPS in the cCRT group or in the drug-therapy group (Supplementary Fig. 2A).
In the cCRT group, PD-L1 expression was significantly increased after treatment not only in patients with positive PD-L1 expression at the baseline (baseline TPS, ≥1) but also in patients with negative PD-L1 expression at the baseline (baseline TPS, 0) (Supplementary Fig. 2B). There was no significant difference in the post-cCRT TPS between patients with negative baseline PD-L1 expression and those with positive baseline PD-L1 expression (median post-cCRT TPS, 30.0 versus 60.0; P = 0.117). PD-L1 expression was significantly increased after cCRT regardless of pathologic response (Supplementary Fig. 2C). There was no significant difference in baseline or post-cCRT TPS according to pathologic response (median baseline TPS, 0.0 in therapeutic effect 1 group and 1.0 for therapeutic effect 2 group P = 0.081; median post-cCRT TPS, 70.0 and 40.0, respectively; P = 0.052).
Alteration in stromal CD8 + TIL density
After cCRT, 22 patients (95.7%) showed an increase in CD8 + TIL density, and only one patient showed a decrease. After drug therapy, 16 patients showed an increase in CD8 + TIL density, and only two patients showed a decrease. CD8 + TIL density was significantly increased after cCRT (median density, 10.6 at the baseline versus 39.1 after cCRT; P < 0.001) and was also increased after drug therapy (11.5 at the baseline versus 22.7 after drug therapy; P = 0.003) (Fig. 2). The post-treatment CD8 + TIL density was significantly higher in the CRT group than in the drug-therapy group (median, 39.1 and 22.7; P = 0.001), whereas baseline CD8 + TIL density was similar in both groups (median, 10.6 and 11.5, respectively; P = 0.874). There was no significant correlation between baseline CD8 + TIL density and post-treatment CD8 + TIL density in the cCRT group or in the drug-therapy group (Supplementary Fig. 3A).
In the cCRT group, CD8 + TIL density was significantly increased after treatment regardless of pathologic response (Supplementary Fig. 3B). However, post-cCRT CD8 + TIL density was significantly higher in therapeutic effect 2 group than in therapeutic effect 1 group (P = 0.001), whereas no significant difference in baseline CD8 + TIL density between two groups was observed (P = 0.309).
Prognosis
Prognostic implication of tumoural PD-L1 expression status and stromal CD8 + TIL density was assessed in 23 patients who underwent cCRT followed by surgery. ROC curve analyses revealed that only the post-cCRT CD8 + TIL density provided a significant prognostic value to predict death or tumour recurrence with the optimal cut-off value of 40 (Fig. 3). The change in TPS or in CD8 + TIL density after cCRT provided no significant prognostic value (P = 1.000 for change in TPS, P = 0.488 for change in CD8 + TIL density).
Receiver operating characteristics curve for programmed death ligand-1 (PD-L1) expression on tumour cells or density of stromal CD8-positive tumour-infiltrating lymphocytes to predict death or recurrence after surgery following concurrent chemo-radiotherapy. PD-L1 programmed death ligand-1, TPS tumour proportion score, cCRT concurrent chemo-radiotherapy, CD8 + TIL CD8-positive tumour-infiltrating lymphocyte, AUC area under curve
Patients with higher post-cCRT CD8 + TIL density (≥40) showed significantly favourable RFS and OS than those with lower post-cCRT CD8 + TIL density (Figs. 4 and 5). For other parameters, RFS and OS were analysed using the median value (1 for baseline TPS, 50 for post-cCRT TPS and 10 for baseline CD8 + TIL density, respectively), which provided no significant prognostic value (Figs. 4 and 5).
Recurrence-free survival curve after surgery following concurrent chemo-radiotherapy according to programmed death ligand-1 (PD-L1) expression on tumour cells or density of CD8-positive tumour-infiltrating lymphocytes. PD-L1 programmed death ligand-1, TPS tumour proportion score, cCRT concurrent chemo-radiotherapy, CD8 + TIL CD8-positive tumour-infiltrating lymphocyte
Overall survival curve after surgery following concurrent chemo-radiotherapy according to programmed death ligand-1 (PD-L1) expression on tumour cells or density of stromal CD8-positive tumour-infiltrating lymphocytes. PD-L1 programmed death ligand-1, TPS tumour proportion score, cCRT concurrent chemo-radiotherapy, CD8 + TIL CD8-positive tumour-infiltrating lymphocyte
Discussion
The present study first demonstrated a significant up-regulation of PD-L1 expression on tumour cells after cCRT in paired clinical samples, which was documented regardless of PD-L1 expression status before cCRT. In addition, the stromal CD8 + TIL density was also significantly increased after cCRT, which was correlated with a higher pathologic response and with a favourable prognosis.
CD8 is a transmembrane glycoprotein that serves as a co-receptor for the T cell receptor (TCR). CD8 is predominantly expressed on cytotoxic T cells (CTLs) that play central roles in cell-mediated immune attack, whereas it can be expressed on natural killer and dendritic cells.15,16,17 Accordingly, CD8 is generally recognised as a marker of CTLs. A number of clinical studies revealed that a higher density of CD8 + TILs was associated with a favourable prognosis in a variety of malignant tumours including NSCLC,17,18,19,20,21,22 The present study also showed that a higher stromal CD8 + TIL density after cCRT was associated with a favourable prognosis (Figs. 3–5). In the present study, we first demonstrated a significant increase in CD8 + TIL density after cCRT (Fig. 2). In addition, of noted, the baseline CD8 + TIL density was not correlated with the post-cCRT CD8 + TIL density (Supplementary Fig. 3A), or provided no significant prognostic value (Figs. 3–5). Accordingly, before initiation of cCRT, it may be difficult to predict CD8 + TIL density after cCRT, which is a significant prognostic marker.
In the present study, higher stromal CD8 + TIL density after cCRT was associated with a higher pathologic response (therapeutic effect 2), which is reasonably explained by experimental results showing that CTLs play a crucial role in anti-tumour effects of radiation.23,24 It has been generally considered that the main mechanism of killing tumour cells by ionising radiation is the direct DNA damage. However, several preclinical studies have revealed that radiation therapy may induce anti-tumour immune response through promoting release of neo-antigen from tumour cells.23,25,26,27 For example, Takeshima and co-workers showed that tumour-inhibitory effect of radiation was almost completely abolished in tumour-bearing mice when CD8-positive T cells were depleted.23 These preclinical results suggest that the immune attack by CD8 + TILs is an important mechanism of anti-tumour effect of radiation therapy. Therefore, when irradiated, tumour cells may evade from immune attack by CD8 + TILs through several mechanisms, which leads to resistance to radiotherapy. The PD-1/PD-L1 axis is the most important mechanism responsible for immune evasion of tumour cells.28 PD-L1 expressed on tumour cells binds to PD-L1 on CTLs, which results in down-regulation of immune activity of CTLs. PD-L1 on tumour cells may be up-regulated in response to immune attack induced by radiotherapy, which has been demonstrated in preclinical models.7,8 The present study is the first clinical study showing up-regulation of PD-L1 after cCRT (Fig. 1). PD-L1 expression may be drastically changed during clinical course especially through a variety of treatment such as radiotherapy and chemotherapy.29 In the present study, pre-treatment PD-L1 status was not correlated with post-treatment PD-L1 status, indicating that post-treatment PD-L1 expression cannot be predicted by pre-treatment PD-L1 status. These results may provide a rationale of PD-L1 blockade following cCRT regardless of pre-treatment PD-L1 status. In fact, consolidation durvalumab treatment following cCRT significantly prolonged progression-free survival and OS in locally advanced NSCLC regardless of baseline PD-L1 status (either for TPS < 25 or for TPS ≥ 25).3,4 In the present study, the majority of patients showed an increase in TPS after cCRT (Fig. 1). Accordingly, we could not compare clinical and pathological characteristics between patients with increased TPS and those with decreased TPS to reveal predictive factors of up-regulation of TPS after cCRT, which should be examined in a large-scale study.
The present study has several limitations due to a variety of weakness. First, this study was a retrospective single-institutional study of a small number of patients. We employed ROC curve analyses to determine the optimal cut-off values of TPS for PD-L1 expression and stromal CD8 + TIL density in prediction of death or tumour recurrence, which may not be suitable for the present study with a small number of patients. The optimal cut-off values should be examined in a large-scale study. Second, the PD-L1 IHC assay employed in the present study is not a companion or complementary diagnostic assay approved for clinical use but is a laboratory-developed test using an antibody against PD-L1 (clone E1L3N). A prospective multi-institutional study revealed that the E1L3N assay showed similar results to trial-validated PD-L1 IHC assays (28–8 and 22C3 assays).12 However, results in the present study shall be validated in a prospective multi-institutional study with a validated IHC assay. Third, there are vast heterogeneity in patient characteristics such as radiation dose and chemotherapy regimen employed in cCRT. The conflicting results between the present study showing increased PD-L1 expression after cCRT and in the previous study showing decreased PD-L1 expression9 may be caused by differences in several patient characteristics. Tumoural PD-L1 status is employed in routine clinical practice as a predictive marker in blockade of PD-1/PD-L1 axis for advanced NSCLC.29,30,31 However, the prognostic value of PD-L1 status remains controversial, and some studies indicated a poor prognosis in patients with strong PD-L1 expression but others indicated a favourable prognosis.13,29,32,33 In the present study, pre-treatment or post-treatment PD-L1 status provided no significant prognostic value in patients who underwent cCRT followed by surgery, whereas high TPS seemed to be associated with a favourable OS (Fig. 5). The prognostic implication should be also assessed and validated in a future prospective study. In addition, 18 patients in the drug-therapy group were treated with a wide variety of regimens including tyrosine kinase inhibitors (TKIs) prescribed for six patients. Cytotoxic agents and TKIs show anti-tumour activity through different mechanism of action and may potentially cause different immune responses. Due to the small number of eligible patients in the study, we did not compare tumour microenvironment between patients treated with cytotoxic agents and those treated with TKIs in the present study, which will be examined in a future study. Finally, the present study provided no data on clinical response to anti-PD-L1 treatment following cCRT. A future study to investigate the effect of post-cCRT tumoural PD-L1 expression status and stromal CD8 + TIL density on anti-PD-L1 treatment following CRT should be conducted.
In conclusion, PD-L1 expression on tumour cells and stromal CD8 + TIL density were significantly increased after cCRT. There was no significant correlation between pre-treatment and post-treatment tumoural PD-L1 or stromal CD8 + TIL density. The higher CD8 + TIL density after cCRT was associated with a higher pathologic response and with a favourable survival.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 68, 7–30 (2018).
Aupérin, A., Le Péchoux, C., Rolland, E., Curran, W. J., Furuse, K., Fournel, P. et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol. 28, 2181–2190 (2010).
Antonia, S. J., Villegas, A., Daniel, D., Curran, W. J., Furuse, K., Fournel, P. et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N. Engl. J. Med. 377, 1919–1929 (2017).
Antonia, S. J., Villegas, A., Daniel, D., Vicente, D., Murakami, S., Hui, R. et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 379, 2342–2350 (2018).
Brahmer, J. R., Govindan, R., Anders, R. A., Antonia, S. J., Sagorsky, S., Davies, M. J. et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment non-small cell lung cancer (NSCLC). J. Immunother. Cancer 6, 75 (2018).
Zhang, P., Su, D. M., Liang, M. & Fu, J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol. Immunol. 45, 1470–1476 (2008).
Deng, L., Liang, H., Burnette, B., Beckett, M., Darga, T., Weichselbaum, R. R. et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 124, 687–695 (2014).
Dovedi, S. J., Adlard, A. L., Lipowska-Bhalla, G., McKenna, C., Jones, S., Cheadle, E. J. et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 74, 5458–5468 (2014).
Fujimoto, D., Uehara, K., Sato, Y., Sakanoue, I., Ito, M., Teraoka, S. et al. Alteration of PD-L1 expression and its prognostic impact after concurrent chemoradiation therapy in non-small cell lung cancer patients. Sci. Rep. 7, 11373 (2017).
Goldstraw, P., Chansky, K., Crowley, J., Rami-Porta, R., Asamura, H., Eberhardt, W. E. et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 11, 39–51 (2016).
Tanaka, F., Yokomise, H., Soejima, T., Uramoto, H., Yamanaka, T., Nakagawa, K. et al. Induction chemoradiotherapy (50 Gy), followed by resection, for stage IIIA-N2 non-small cell lung cancer. Ann. Thorac. Surg. 106, 1018–1024 (2018).
Rimm, D. L., Han, G., Taube, J. M., Yi, E. S., Bridge, J. A., Flieder, D. B. et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 3, 1051–1058 (2017).
Hirai, A., Yoneda, K., Shimajiri, S., Kuroda, K., Hanagiri, T., Fujino, Y. et al. Prognostic impact of programmed death-ligand 1 expression in correlation with human leukocyte antigen class I expression status in stage I adenocarcinoma of the lung. J. Thorac. Cardiovasc Surg. 155, 382–392 (2018).
Donnem, T., Hald, S. M., Paulsen, E. E., Richardsen, E., Al-Saad, S., Kilvaer, T. K. et al. Stromal CD8+T-cell density—a promising supplement to TNM staging in non-small cell lung cancer. Clin. Cancer Res. 21, 2635–2643 (2015).
Klebanoff, C. A., Gattinoni, L. & Restifo, N. P. CD8þ T-cell memory in tumor immunology and immunotherapy. Immunol. Rev. 211, 214–224 (2006).
Restifo, N. P., Dudley, M. E. & Rosenberg, S. A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 12, 269–281 (2012).
Hendry, S., Salgado, R., Gevaert, T., Russell, P. A., John, T., Thapa, B. et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv. Anat. Pathol. 24, 311–335 (2017).
Remark, R., Becker, C., Gomez, J. E., Damotte, D., Dieu-Nosjean, M., C. Sautès-Fridman, C. et al. The non-small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome. Am. J. Respir. Crit. Care Med. 191, 377–390 (2015).
Donnem, T., Kilvaer, T. K., Andersen, S., Richardsen, E., Paulsen, E. E., Hald, S. M. et al. Strategies for clinical implementation of TNM-Immunoscore in resected nonsmall-cell lung cancer. Ann. Oncol. 27, 225–232 (2016).
Geng, Y., Shao, Y., He, W., Hu, W., Xu, Y., Chen, J. et al. Prognostic role of tumor-infiltrating lymphocytes in lung cancer: a meta-analysis. Cell Physiol. Biochem. 37, 1560–1571 (2015).
Zeng, D. Q., Yu, Y. F., Ou, Q. Y., Li, X. Y., Zhong, R. Z., Xie, C. M. et al. Prognostic and predictive value of tumor-infiltrating lymphocytes for clinical therapeutic research in patients with non-small cell lung cancer. Oncotarget 7, 13765–13781 (2016).
Tokito, T., Azuma, K., Kawahara, A., Ishii, H., Yamada, K., Matsuo, N. et al. Predictive relevance of PD-L1 expression combined with CD8+TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur. J. Cancer 55, 7–14 (2016).
Takeshima, T., Chamoto, K., Wakita, D., Ohkuni, T., Togashi, Y., Shirato, H. et al. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res. 70, 2697–2706 (2010).
Chen, H. Y., Xu, L., Li, L. F., Liu, X. X., Gao, J. X. & Bai, Y. R. Inhibiting the CD8+T cell infiltration in the tumor microenvironment after radiotherapy is an important mechanism of radioresistance. Sci. Rep. 8, 11934 (2018).
Lugade, A., Moran, J., Gerber, S., Rose, R., Frelinger, J. & Lord, E. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 174, 7516–7523 (2005).
Schaue, D., Comin-Anduix, B., Ribas, A., Zhang, L., Goodglick, L., Sayre, J. W. et al. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin. Cancer Res. 14, 4883–4890 (2008).
Lee, Y., Auh, S. L., Wang, Y., Burnette, B., Wang, Y., Meng, Y. et al. Therapeutic effects of ablative radiation on local tumor require CD8+T cells: changing strategies for cancer treatment. Blood 114, 589–595 (2009).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Yu, H., Boyle, T. A., Zhou, C., Rimm, D. L. & Hirsch, F. R. PD-L1 expression in lung cancer. J. Thorac. Oncol. 11, 964–975 (2016).
Reck, M., Rodríguez-Abreu, D., Robinson, A. G., Csőszi, T., Fülöp, A., Gottfried, M. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. c75, 1823–1833 (2016).
Hanna, N., Johnson, D., Temin, S., Baker, S. Jr, Brahmer, J., Ellis, P. M. et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical practice guideline update. J. Clin. Oncol. 35, 3484–3515 (2017).
Soo, R. A., Chen, Z., Yan Teng, R. S., Tan, H. L., Iacopetta, B., Tai, B. C. et al. Prognostic significance of immune cells in non-small cell lung cancer: meta-analysis. Oncotarget 9, 24801–24820 (2018).
Imanishi, N., Hirai, A., Yoneda, K., Shimajiri, S., Kuwata, T., Tashima, Y. et al. Programmed death-ligand 1 (PD-L1) expression in pleomorphic carcinoma of the lung. J. Surg. Oncol. 117, 1563–1569 (2018).
Acknowledgements
We thank all doctors in the University of Occupational and Environmental Health Japan (Second Department of Surgery, Department of Respiratory Medicine, Department of Pathology and Oncology and Department of Pathology) for helpful assistance in collecting and processing clinical samples. We also thank Dr. Yoshiyuki Fujino (Professor, Department of Environmental Epidemiology, Institute of Industrial Ecological Sciences, University of Occupational and Environmental Health, Japan), an expert in medical statistics, for helpful comments on statistical analyses in the study.
Author information
Authors and Affiliations
Contributions
K.Y. and F.T. participated in the study conception and design, data collection, data analysis, reviewed the paper and approved the final draft for submission. T.K., M.K., M.M. and T.K. participated in data collection, reviewed the paper and approved the final draft for submission. K.Y., T.O., M.H. and T.N. participated in data analysis, reviewed the paper and approved the final draft for submission.
Corresponding author
Ethics declarations
Competing interests
K.Y. reports grants from ASAHI KASEI PHARMA CORPORATION, grants and personal fees from Astellas Pharma Inc., grants and personal fees from AstraZeneca K.K., grants and personal fees from Bristol-Myers Squibb K.K., grants and personal fees from CHUGAI PHARMACEUTICAL CO., LTD., grants from DAIICHI SANKYO COMPANY, LIMITED, grants from Daiwa Securities Health Foundation, grants and personal fees from Eli Lilly Japan K.K., grants from FUJIFILM Pharma Co., Ltd., grants from Fukuda Denshi Co., Ltd., grants and personal fees from GlaxoSmithKline K.K., grants from KAKENHI (Grants-in-Aid for Scientific Research) (C), grants from KYORIN Pharmaceutical Co.,Ltd., grants from Kyowa Hakko Kirin Co., Ltd., grants from Meiji Seika, grants and personal fees from MSD K.K. a subsidiary of Merck & Co.,Inc., grants from Mylan Inc., grants from Nippon Boehringer lngelheim Co., Ltd., grants from North East, grants and personal fees from Novartis Pharma K.K., grants from ONO PHARMACEUTICAL CO., LTD., grants from Oxford Immunotec, Inc., grants and personal fees from Pfizer Inc., grants and personal fees from Shionogi & Co., Ltd., grants from Sumitomo Dainippon Pharma Co., Ltd., grants and personal fees from TAIHO Pharmaceutical Co., Ltd., grants and personal fees from Taisho Toyama Pharmaceutical Co., Ltd., grants and personal fees from TEIJIN HOME HEALTHCARE LIMITED., outside the submitted work. F.T. reports grants and personal fees from ASAHI KASEI PHARMA CO, grants from Astellas Pharma Inc., grants and personal fees from AstraZeneca K.K., personal fees from Bristol-Myers Squibb K.K., grants and personal fees from CHUGAI PHARMACEUTICAL CO., LTD., grants and personal fees from Eli Lilly Japan K.K., grants and personal fees from Kyowa Hakko Kirin Co., Ltd., grants and personal fees from MSD K.K., grants and personal fees from Novartis Pharma K.K., grants and personal fees from ONO PHARMACEUTICAL CO., LTD., personal fees from Pfizer Inc., grants and personal fees from Shionogi & Co., Ltd., grants and personal fees from TAIHO Pharmaceutical Co., Ltd., grants from Eizai Co. Ltd., grants and personal fees from Nippon Boehringer Ingelheim Co. Ltd., personal fees from Johnson & Johnson Co. Ltd., personal fees from Covidien Japan Co. Ltd., outside the submitted work. The remaining authors declare no competing interests.
Ethics approval and consent to participate
The institutional review board of the University of Occupational and Environmental Health, Japan approved the present study. This study was performed in accordance with the Declaration of Helsinki.
Funding
This study was supported in part by the Japan Society for the Promotion of Science (JSPS) [Grants-in-Aid for Scientific Research Grant Numbers 16H10697 and 16H01747], UOEH Research Grant for Promotion of Occupational Health.
Consent to publish
Not applicable.
Data availability
Requests for data and reagents can be made by contacting the corresponding author.
Note
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoneda, K., Kuwata, T., Kanayama, M. et al. Alteration in tumoural PD-L1 expression and stromal CD8-positive tumour-infiltrating lymphocytes after concurrent chemo-radiotherapy for non-small cell lung cancer. Br J Cancer 121, 490–496 (2019). https://doi.org/10.1038/s41416-019-0541-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-019-0541-3
This article is cited by
-
Impact of platinum-based chemotherapy on the tumor mutational burden and immune microenvironment in non-small cell lung cancer with postoperative recurrence
Clinical and Translational Oncology (2024)
-
Radiation combined with immune checkpoint inhibitors for unresectable locally advanced non-small cell lung cancer: synergistic mechanisms, current state, challenges, and orientations
Cell Communication and Signaling (2023)
-
PET-based radiomics visualizes tumor-infiltrating CD8 T cell exhaustion to optimize radiotherapy/immunotherapy combination in mouse models of lung cancer
Biomarker Research (2023)
-
Impact of TMB/PD-L1 expression and pneumonitis on chemoradiation and durvalumab response in stage III NSCLC
Nature Communications (2023)
-
Impact of adjuvant chemotherapy and radiotherapy on tumour-infiltrating lymphocytes and PD-L1 expression in metastatic breast cancer
British Journal of Cancer (2023)