Abstract
Background
Proteinuria monitoring is required in patients receiving lenvatinib, however, current methodology involves burdensome overnight urine collection.
Methods
To determine whether the simpler urine protein:creatinine ratio (UPCR) calculated from spot urine samples could be accurately used for proteinuria monitoring in patients receiving lenvatinib, we evaluated the correlation between UPCR and 24-hour urine protein results from the phase 3 REFLECT study. Paired data (323 tests, 154 patients) were analysed.
Results
Regression analysis showed a statistically significant correlation between UPCR and 24-hour urine protein (R2: 0.75; P < 2 × 10−16). A UPCR cut-off value of 2.4 had 96.9% sensitivity, 82.5% specificity for delineating between grade 2 and 3 proteinuria. Using this UPCR cut-off value to determine the need for further testing could reduce the need for 24-hour urine collection in ~74% of patients.
Conclusion
Incorporation of UPCR into the current algorithm for proteinuria management can enable optimisation of lenvatinib treatment, while minimising patient inconvenience.
Clinical trial registration
NCT01761266
Similar content being viewed by others
Background
Lenvatinib is a multikinase inhibitor of vascular endothelial growth factor receptors (VEGFR) 1‒3, fibroblast growth factor receptors 1–4, platelet-derived growth factor-alpha, KIT, and RET.1,2 Lenvatinib monotherapy is indicated for first-line treatment of unresectable hepatocellular carcinoma (HCC)3,4 based on results from the phase 3 REFLECT study, where lenvatinib demonstrated a treatment effect on overall survival with statistical confirmation of noninferiority to sorafenib (13.6 vs 12.3 months, respectively; hazard ratio 0.92; 95% confidence interval 0.79–1.06), along with significant improvements in progression-free survival, time to progression, and objective response rates.5 Lenvatinib is also indicated as monotherapy for patients with locally recurrent or metastatic, progressive, radioiodine-refractory differentiated thyroid cancer (RR-DTC), and in combination with everolimus in patients with advanced renal cell carcinoma (RCC) following 1 prior anti-angiogenic therapy.3
Proteinuria is a class effect of antiangiogenic agents,6 and a well-documented lenvatinib-associated adverse effect.5,7,8,9 Rates of any-grade/grade ≥3 proteinuria observed in lenvatinib-treated patients were 25%/6% in the phase 3 REFLECT study in unresectable HCC,5 31%/10% in the phase 3 SELECT in patients with RR-DTC,8 and 31%/19% in a phase 2 study in patients with advanced/metastatic RCC.7
Patients receiving lenvatinib are monitored regularly for proteinuria using a urine sample dipstick method. The current standard management requires a 24-hour urine protein test if a dipstick proteinuria result of ≥2+ is detected, with the recommendation that lenvatinib treatment should be withheld if a proteinuria level of ≥2 g/24 h is detected.3 This 24-hour urine protein test relies on patient collection of urine overnight, which is burdensome and may be influenced by patient compliance. However, the single (“spot”) urine protein:creatinine ratio (UPCR) is a simple and convenient alternative test that is often used to detect proteinuria associated with certain medical conditions, e.g., chronic kidney disease and diabetes.10,11 UPCR is calculated by dividing the level of protein (mg/dl) in a spot urine test by the creatinine level (mg/dl).12 This approach was first validated in 1983 by Ginsberg and colleagues.13 It is based on the premise that urinary creatinine excretion and the protein excretion rate in the presence of a stable glomerular filtration rate are fairly constant in a given patient. Thus, a simple ratio of the 2 in a single-void urine sample would reflect cumulative protein excretion over a day (because the ratio of 2 stable rates would cancel out the time factor).
To determine whether UPCR could be a useful and more convenient assessment for proteinuria in patients receiving lenvatinib, we evaluated the correlation between proteinuria assessment by UPCR and 24-hour urine protein in patients with HCC from the REFLECT study.
Materials and methods
Study design and patients
Details of the REFLECT study have been published previously.5 Briefly, REFLECT was an international, randomised, open-label, noninferiority study, which enroled 954 previously untreated patients with unresectable HCC from 154 sites in 20 countries throughout Asia-Pacific, European, and North American regions. Patients were randomised (1:1) to receive lenvatinib (n = 478) or sorafenib (n = 476).
End points and assessments
All patients underwent urine dipstick testing at each scheduled safety assessment visit (day 1 and day 15 of the first 2 cycles, then day 1 of each subsequent cycle). Within 72 h of a positive (≥2) proteinuria urine dipstick test during regular safety assessment visits, patients underwent standard-of-care 24-hour urine collection for total protein, performed either at the central or local site laboratories, as well as a UPCR test, performed at the central laboratory. 24-Hour urine collection was used to grade proteinuria according to Common Terminology Criteria for Adverse Events (CTCAE) v4.0 criteria (Supplementary Table 1). Patients with positive urine dipstick test underwent dipstick testing every 2 weeks until results were reduced to 1+ or negative for 3 consecutive months.
Statistical analyses
Paired data were analysed. Optimal UPCR cut-off values were identified using standard receiver operating characteristic methods to maximise the Youden index/statistics (sensitivity + specificity), with urine data dichotomised by CTCAE proteinuria grades (grade <2 vs ≥2 and grade ≤2 vs 3). The correlation between 24-hour urine protein data (as continuous values) and UPCR was analysed using a regression model of log-transformed data. Also, the optimal UPCR cut-off value determining which patients should undergo further testing with 24-hour urine collection was calculated. Statistical analyses were performed using R statistical software.
Results
Paired data (323 paired tests) from 154 patients were included in the analysis.
Cut-off values for UPCR
The optimal cut-off to discriminate grade 1 (1+ proteinuria; urinary protein <1.0 g/24 h) from grade ≥2 (2+ proteinuria; urinary protein >1.0 g/24 h) proteinuria by UPCR was 1.02, (94.0% sensitivity; 72.4% specificity) (Supplementary Fig. 1A). The optimal cut-off to discriminate grade 2 from grade 3 proteinuria by UPCR was 2.43 (96.9% sensitivity; 82.5% specificity) (Supplementary Fig. 1B), the positive likelihood ratio was 5.54, and the negative likelihood ratio was 0.038. For ease of clinical use, a cut-off of 2.4 is proposed.
Correlation between 24-hour urine protein collection and UPCR
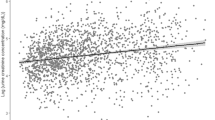
After removal of a single outlier from the regression model, regression analysis of UPCR versus 24-hour urine protein collection data showed a statistically significant correlation, with a Pearson correlation coefficient (R) of 0.86, an R2 of 0.75, and slope of 0.9 (P < 2 × 10−16) (Fig. 1a). Below the grade 3 optimal UPCR cut-off level (2.4), only 1 test out of 239 had grade 3 proteinuria based on 24-hour urine collection but not based on UPCR (Fig. 1a). Using the grade 1 + 2 versus grade 3 proteinuria UPCR cut-off of 2.4 as an optimal cut-off to determine which patients should undergo further testing with 24-hour urine collection, 239/323 pairs of tests were below or equal to this cut-off and only 1 test was missed (0.4%); thus, the need for 24-hour urine collection would be reduced by 74% (Fig. 1b). Also, 84/323 (26%) pairs of tests were above this cut-off, with 53/84 (63%) pairs being grade 1 + 2 proteinuria.
a Correlation between 24-hour urine protein collection and UPCR, analysed using a regression model of log-transformed data (323 paired tests). The red arrow indicates a single test that had grade 3 proteinuria based on 24-hour urine collection but not with UPCR based on the optimal UPCR cut-off value of 2.4; b proposed UPCR cut-off values for determining whether to perform 24-hour urine collection. Units of measurement are mg/dl for urine protein, and ratio for UPCR. Ln, natural log; UPCR, urine protein:creatinine ratio
Discussion
The mechanism underlying VEGFR-inhibitor-associated proteinuria are unclear, but may involve thrombotic microangiopathy or impairment of podocyte function.14 However, proteinuria is a manageable adverse event that does not typically lead to clinically meaningful adverse outcomes if it is appropriately monitored and managed.3,14 The regression model of the UPCR versus 24-hour urine protein data suggests a strong and statistically significant correlation between the 2 measurements, and using a UPCR cut-off value of 2.4 to delineate between grade 2 and 3 proteinuria and the need for further testing would obviate the need for the more burdensome 24-hour urine collection for an estimated 74% of patients with urine protein dipstick results of ≥2+.
Introduction of UPCR into the guidelines for proteinuria management could be as follows and as described in Supplementary Table 2: urine dipstick testing would be performed as regularly scheduled. A 24-hour urine collection or an immediate spot UPCR test would be required in the case of: (1) first occurrence of ≥2+ proteinuria while on lenvatinib; (2) a subsequent increase in severity of urine dipstick proteinuria while on the same dose level; (3) when following a lenvatinib dose reduction, the urine protein dipstick result was ≥2+. In addition, a 24-hour urine collection should be initiated as soon as possible (within 72 h) when UPCR is ≥2.4 to verify the grade of proteinuria. After the proteinuria has improved to a lower grade, lenvatinib may be restarted at a reduced dose. By following these criteria, proteinuria can be safely managed, enabling optimisation of lenvatinib treatment while minimising inconvenience to patients. It should also be noted that, although this analysis was conducted in patients with HCC, this monitoring approach may potentially be useful in other tumour types treated with lenvatinib (e.g. differentiated thyroid cancer or renal cell carcinoma).
Conclusions
Data from this large study support the use of UPCR for proteinuria monitoring in patients with unresectable HCC who are receiving lenvatinib therapy and have ≥2+ urine protein during routine monitoring, similar to its use in other diseases. Use of this test would alleviate the need for 24-hour urine collection in most cases, therefore reducing patient burden.
Change history
30 July 2019
This article was originally published under a standard license to Publish, but has now been made available under a CC BY license. The PDF and HTML versions of the paper have been modified accordingly.
An amendment to this paper has been published and can be accessed via a link at the top of the paper
References
Matsui, J., Yamamoto, Y., Funahashi, Y., Tsuruoka, A., Watanabe, T., Wakabayashi, T. et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer 122, 664–671 (2008).
Okamoto, K., Kodama, K., Takase, K., Sugi, N. H., Yamamoto, Y., Iwata, M. et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett 340, 97–103 (2013).
Lenvima (lenvatinib) [prescribing information] (Woodcliff Lake, NJ: Eisai Inc., 2018).
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Hepatobiliary Cancers. Version 2.2019, https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (accessed 18 March 2019).
Kudo, M., Finn, R. S., Qin, S., Han, K. H., Ikeda, K., Piscaglia, F. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173 (2018).
Launay-Vacher, V. & Deray, G. Hypertension and proteinuria: a class-effect of antiangiogenic therapies. Anticancer Drugs 20, 81–82 (2009).
Motzer, R. J., Hutson, T. E., Glen, H., Michaelson, M. D., Molina, A., Eisen, T. et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 16, 1473–1482 (2015).
Schlumberger, M., Tahara, M., Wirth, L. J., Robinson, B., Brose, M. S., Elisei, R. et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372, 621–630 (2015).
Cavalieri, S., Cosmai, L., Genderini, A., Nebuloni, M., Tosoni, A., Favales, F. et al. Lenvatinib-induced renal failure: two first-time case reports and review of literature. Expert Opin Drug Metab Toxicol 14, 379–385 (2018).
Methven, S., MacGregor, M. S., Traynor, J. P., O’Reilly, D. S. & Deighan, C. J. Assessing proteinuria in chronic kidney disease: protein-creatinine ratio versus albumin-creatinine ratio. Nephrol Dial Transplant 25, 2991–2996 (2010).
Biradar, S. B., Kallaganad, G. S., Rangappa, M., Kashinakunti, S. V. & Retnakaran, R. Correlation of spot urine protein-creatinine ratio with 24-hour urinary protein in type 2 diabetes mellitus patients: a cross sectional study. J Res Med Sci 16, 634–639 (2011).
Yang, C. Y., Chen, F. A., Chen, C. F., Liu, W. S., Shih, C. J., Ou, S. M. et al. Diagnostic accuracy of urine protein/creatinine ratio is influenced by urine concentration. PLoS One 10, e0137460 (2015).
Ginsberg, J. M., Chang, B. S., Matarese, R. A. & Garella, S. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med 309, 1543–1546 (1983).
Takahashi, S., Kiyota, N. & Tahara, M. Optimal use of lenvatinib in the treatment of advanced thyroid cancer. Cancers Head Neck 2, 7 (2017).
Acknowledgements
Medical writing assistance was provided by Suhaida A. Selamat, PhD, Oxford PharmaGenesis Inc., Newtown, PA, USA. Presented in part at the International Liver Cancer Association 12th Annual Conference, 14–18 September 2018, London, United Kingdom.
Author contributions
TRJE: Helped conceive the design of the study, collected data, interpreted data, critically reviewed the manuscript drafts, and provided final approval of the manuscript to be published. MK: Helped conceive the design of the study, collected data, interpreted data, critically reviewed the manuscript drafts, and provided final approval of the manuscript to be published. RSF: Helped conceive the design of the study, collected data, interpreted data, critically reviewed the manuscript drafts, and provided final approval of the manuscript to be published. K-HH: Helped conceive the design of the study, collected data, interpreted data, critically reviewed the manuscript drafts, and provided final approval of the manuscript to be published. A-LC: Helped conceive the design of the study, collected data, interpreted data, critically reviewed the manuscript drafts, and provided final approval of the manuscript to be published. MI: Helped conceive the design of the study, collected data, interpreted data, critically reviewed the manuscript drafts, and provided final approval of the manuscript to be published. SK: Analysed data, interpreted data, critically reviewed the manuscript drafts, and provided final approval of the manuscript to be published. MR: Analysed data, interpreted data, critically reviewed the manuscript drafts, and provided final approval of the manuscript to be published. CED: Helped conceive the design of the study, interpreted data, critically reviewed the manuscript drafts, and provided final approval of the manuscript to be published. FP: Helped conceive the design of the study, collected data, interpreted data, critically reviewed the manuscript drafts, and provided final approval of the manuscript to be published. MWS: Helped conceive the design of the study, collected data, interpreted data, critically reviewed the manuscript drafts, and provided final approval of the manuscript to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
TRJE: Reports honoraria from Eisai for advisory boards and speaker’s bureau (payable to his institution); support from Eisai for sponsored clinical trials (payable to the institution); honoraria for advisory boards and speaker’s bureau from Bristol-Myers Squibb, Bayer, GlaxoSmithKline, Roche/Genentech (all payable to his institution); honoraria for advisory boards from Celgene, Karus Therapeutics, Baxalta, TC BioPharm, Immunova (all payable to his institution); support for sponsored clinical trials (payable to the institution) from Bristol-Myers Squibb, GlaxoSmithKline, Roche / Genentech, Celgene, TC BioPharm, Merck, Novartis, e-Therapeutics, Vertex, Verastem, Daiichi, AstraZeneca, Basilea, Immunocore, Chugai; support to attend international scientific conferences from Bristol-Myers Squibb, Roche/Genentech, Bayer, Merck, Eisai; he is co-editor of the clinical subjects section of the British Journal of Cancer. MK: Reports grants/research support from Bayer, Daiichi Sankyo, Chugai, Otsuka, Taiho, Sumitomo Dainippon, and Merck Sharp & Dohme Corp; received honoraria from Bayer, Eisai, Merck Sharp & Dohme Corp, BMS, and EA Pharma; and consulting/advisory role for Bayer and Eisai. RSF: Reports grants/research support from Bayer, BMS, Novartis, Pfizer, Eisai, Eli Lilly, and Merck (payable to his institution); provided expert testimony for Novartis; and consulting/advisory role for AstraZeneca, Bayer, BMS, Eisai, Eli Lilly, Novartis, Merck, Pfizer, and Roche/Genentech. K-HH: Reports grants/research support from Eisai and Kowa; and served as a consultant for Eisai, Kowa, and Bayer. A-LC: Reports personal fees for consulting/advisory role for BMS, Ono, Novartis, Bayer, Merck, and Merck Sharp & Dohme Corp. MI: Reports consulting/advisory role for Bayer Yakuhin, Eisai, Novartis Pharma, Shire and MSD; research support from Bayer Yakuhin, Kyowa Hakko Kirin, Yakult, Eli Lilly Japan, Ono Pharmaceutical, Eisai, AstraZeneca, Baxalta Japan Limited, Chugai Pharmaceutical, Bristol-Myers Squibb, Merck Serono, Nano Carrier, ASLAN Pharmaceuticals, Novartis Pharma, and Takara Bio. FP: Reports grant/research support from ESAOTE; received honoraria for advisory/consultant activities from Eisai, AstraZeneca, Bayer, GE, Tiziana Life Sciences; and honoraria for speakers’ bureau from Bayer and Bracco. MWS: Received honoraria for advisory boards from Eisai, Bayer and Exelixis. SK: Former employee of Eisai Ltd. MR and CED are employees of Eisai Inc.
Ethics approval and consent to participate
All relevant institutional review boards approved the protocol for this clinical trial, which was performed in accordance with the Declaration of Helsinki. Ethics committee names are provided in a separate document.
Funding
This study was funded by Eisai Inc., Woodcliff Lake, NJ, and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Medical writing assistance was provided by Suhaida A. Selamat, PhD, of Oxford PharmaGenesis Inc., with funding provided by Eisai Inc.
Consent to publish
No identifying patient information is included in this report. All patients signed informed consent forms for the clinical trial.
Data availability
The datasets generated during and/or analysed during the current study are on file with Eisai and not publicly available.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Evans, T.R.J., Kudo, M., Finn, R.S. et al. Urine protein:creatinine ratio vs 24-hour urine protein for proteinuria management: analysis from the phase 3 REFLECT study of lenvatinib vs sorafenib in hepatocellular carcinoma. Br J Cancer 121, 218–221 (2019). https://doi.org/10.1038/s41416-019-0506-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-019-0506-6
This article is cited by
-
Analysis of Anti-Angiogenesis-Related Adverse Events Associated with Vascular Endothelial Growth Factor Receptor-Tyrosine Kinase Inhibitors (VEGFR-TKIs) in Patients with Metastatic Renal Cell Carcinoma
Targeted Oncology (2023)
-
Decision making for anti-VEGF inhibitor continuation: dip stick? or urine protein/creatinine ratio? (VERSiON UP study)
BMC Cancer (2022)
-
Diagnostic efficacy and influence factors of urinary protein/creatinine ratio replacing 24-h urine protein as an evaluator of proteinuria in children
International Urology and Nephrology (2022)
-
Risk factors of proteinuria and potentially protective effect of renin–angiotensin system inhibitors in patients with renal cell carcinoma receiving axitinib
Cancer Chemotherapy and Pharmacology (2022)
-
Reporting of harms in oncological clinical study reports submitted to the European Medicines Agency compared to trial registries and publications—a methodological review
BMC Medicine (2021)