Abstract
Background
Older patients have increased risk of toxicity from chemotherapy. Current prediction tools do not provide information on cumulative risk.
Methods
Patients aged ≥ 70 years with solid cancer were prospectively enrolled. A prediction model was developed for adverse events (AEs) ≥ Grade 3 (G3), based on geriatric assessment (GA), laboratory, and clinical variables.
Results
301 patients were enrolled (median age, 75 years). Median number of chemotherapy cycles was 4. During first-line chemotherapy, 53.8% of patients experienced AEs ≥ G3. Serum protein < 6.7 g/dL, initial full-dose chemotherapy, psychological stress or acute disease in the past 3 months, water consumption < 3 cups/day, unable to obey a simple command, and self-perception of poor health were significantly related with AEs ≥ G3. A predicting model with these six variables ranging 0–8 points was selected with the highest discriminatory ability (c-statistic= 0.646), which could classify patients into four risk groups. Predicted cumulative incidence of AEs ≥ G3 was discriminated according to risk groups.
Conclusions
This prediction tool could identify the risk of AEs ≥ G3 after chemotherapy and provide information on the cumulative incidence of AEs in each cycle.
Clinical Trial Id
WHO ICTRP number, KCT0001071
Similar content being viewed by others
Introduction
Older patients with cancer have distinct characteristics of physical, emotional, cognitive, and nutritional function when compared with younger patients.1 These patients have a decreased capacity for recovery from internal and external stress, and are susceptible to adverse events from cancer treatment.1,2 However, there remains minimal evidence from clinical trials on the efficacy and safety of cancer treatment in older patients.3,4 Therefore, it is challenging to make evidence-based decisions on the use of cytotoxic chemotherapy in older populations.
Geriatric assessment (GA) has been proved to be an objective tool to quantify the overall health status of older populations more comprehensively and precisely.5,6,7 It has been reported that GA could be associated with life expectancy, compliance of chemotherapy, postoperative mortality risk, and early death.5,6,8,9 Two prediction tools for chemotherapy toxicity based on GA have been reported.10,11,12 However, both of these studies were conducted in Western countries. Prevalent cancer types, drug metabolism, nutritional status, and social support are inevitably different according to different countries, races, and cultures.13,14,15,16,17 In addition, ethnic differences in drug metabolism and chemotherapy toxicities are also well established.18,19 Furthermore, the type and number of domains included in GA are diverse between different institutions and societies in clinical practice.20 Therefore, different studies using GA are needed in Asian countries. In addition, two different prediction tools for chemotherapy toxicity predicted dichotomous outcomes regardless of completed chemotherapy cycles.10,12 In routine clinical practice, information on the occurrence of adverse events in each chemotherapy cycle may be more useful. The cumulative incidence of toxicity may provide additional information since incidence of toxicities increase as the chemotherapy cycle proceeds.
Therefore, we aimed to develop a novel prediction tool to predict chemotherapy toxicity in Asian populations using clinical parameters and GA. The cumulative risk of toxicity was explored in proceeding chemotherapy cycles.
Patients and methods
Study design and participants
The Korean Cancer Study Group (KCSG) study PC13-09 was a prospective, longitudinal, and multicentre cohort study to develop a prediction tool for adverse events ≥ Grade 3 (G3) due to chemotherapy. Between February 2014 and December 2015, 301 patients were enrolled in 17 hospitals affiliated with the KCSG. The primary outcome was defined as occurrence of adverse events ≥ G3. Inclusion criteria included the following: patient ≥70 aged old; candidate for first-line palliative chemotherapy; and patients with histologically confirmed solid tumour. The exclusion criteria included the following: haematologic malignancy such as lymphoma, leukaemia, and multiple myeloma; patient who had a treatment plan to receive monotherapy with biologic agent or targeted agent, concurrent chemoradiotherapy, combination chemotherapy with investigational agents, or monotherapy with oral agents; and recurrent cases during adjuvant chemotherapy. GA was conducted after obtaining informed consent and before first-line chemotherapy. Chemotherapy regimen was chosen at an oncologist’s discretion. The dosing of chemotherapy regimen was recommended as described in the National Comprehensive Cancer Network guideline. Initial dose reduction was permitted based on clinical decision by the investigator. Adverse events were assessed by using National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 in each cycle of chemotherapy.
Clinical parameters and GA
Pretreatment baseline measures such as laboratory findings (complete blood cell counts and chemistry), cancer type, stage, and fracture history were recorded. Chemotherapy regimen and dosing were documented. Patients were followed to collect adverse events at the end of each cycle. As in our previous studies, GA consisted of evaluating medical problems, social support, functional status, cognitive status, emotional status, nutritional status, and mobility.5,6,7,21 In brief, comorbidity was measured using the Charlson comorbidity index and was divided into low (0 points), medium (1–2 points), high (3–4 points), and very high (≥5 points) groups according to the original weighting system.22 Functional status was evaluated using the activities of daily living (ADL) and Korean instrument ADL (K-IADL) scores.23,24,25 At least one dependency in ADL or K-IADL was categorised as ADL-dependent or IADL-dependent, respectively. Timed Get Up and Go test (TGUG) >20 s was regarded as impaired mobility.26 Cognitive function was evaluated using Mini Mental Status Examination (MMSE) in the Korean version of the Consortium to Establish a Registry for Alzheimer’s disease Assessment Packet, which was divided into severe cognitive impairment (scores ≤ 16) and mild cognitive impairment (scores 17–24).27 For depression, Short-Form Geriatric Depression Scale scores of 5–9 and of 10 or more indicated mild depression and severe depression, respectively, (ranging from 0 to 15).28 In terms of nutritional status, the Mini Nutritional Assessment (MNA) score <17.0 and between 17.0 and 23.5 indicated malnutrition and a risk for malnutrition, respectively.29 Polypharmacy was evaluated based on number of drugs taken both descriptively and quantitatively. GA was conducted by clinical research coordinators who attended GA workshops to standardise GA and received certificate issued by KCSG.
Development of prediction tool
Variables used to develop a predictive model for chemotherapy toxicity were as follows: clinical parameters such as age, sex, performance status, chemotherapy regimen, initial dose reduction, tumour type; laboratory findings; and all items of each domain in GA. Variables significantly associated with occurrence of adverse events ≥ G3 were identified in univariate analysis using a Cox proportional-hazards model. For developing a prediction tool, selected variables were assigned a score according to hazard ratios for adverse events ≥ G3 in a multivariate analysis of the Cox proportional-hazards model. Compared with actual incidence, the best prediction model was selected based on c-statistic.
Statistical analysis
Assuming an incidence of 45% for adverse events ≥ G3 and drop-out rate of 10%, at least 200 patients were needed based on the incidence of adverse events ≥ G3 estimated in the population by Harrell’s guideline. The protocol was amended for robust significance to include 300 patients. The Cox proportional-hazards model was used to identify variables related with occurrence of adverse events ≥ G3. Multivariate models included variables that showed significance in univariate analysis with p < 0.05. The discriminatory ability of the prediction tool was evaluated using the c-statistic. Predicted probabilities for each cycle were generated based on the risk scores. This study was approved by the institutional review board of each participating centre and the KCSG (KCSG PC13-09). This study was registered with Clinical Research Information Service (WHO ICTRP number: KCT0001071). Patients completed the informed consent process.
Results
Patient characteristics
We enrolled 301 patients aged ≥70 years in this trial. Baseline patient characteristics, including demographics, chemotherapy, laboratory findings, and GA are shown in Tables 1 and 2. Forty-two patients (14.0%) were 80 years or older. Most patients had good performance status with Eastern Cooperative Oncology Group (ECOG) ≤ 1 (81.4%) and stage IV (97.0%). Median body mass index (BMI) was 22.5 (range 14.0–31.2). The most common cancer types were colorectal cancer (28.9%), lung cancer (24.6%), hepato-biliary-pancreatic cancer (22.3%), and stomach cancer (10.6%). In 177 patients (58.8%), initial dose reduction was applied at the first cycle.
In terms of GA, 14.0% and 70.1% of patients lived alone and with a spouse, respectively. The median number of medications taken was 5. According to Charlson comorbidity index, most patients had low or medium risks of comorbidity (52.2% and 37.9%, respectively). ADL and IADL were dependent in 28.6% and 41.2% of patients, respectively. Mild and severe impairment of cognitive function by MMSE were detected in 45.5% and 10.0% of patients, respectively. Mild and severe depression occurred in 30.6% and 13.3% of participants, respectively. The risk of malnutrition and having malnutrition, as assessed by MNA and impaired mobility by TGUG > 20 s, were identified in 56.8%, 19.6%, and 8.3%, respectively. In laboratory findings, low haemoglobin (Hb) level (Hb < 10 g/dL in female and Hb < 11 g/dL in male), hyponatremia (<135 mmol/L), and hypoalbuminemia (<3.6 g/dL) were shown in 23.9%, 22.6%, and 35.2% of patients, respectively.
Chemotherapy and adverse events
The median number of chemotherapy cycles given was four in this study (range 25–75%, 2–7 cycles). On the discretion of the physician and according to tumour type, various chemotherapy regimens were administered in the enrolled patients (Supplementary Table 1). Five patients were not followed up for chemotherapy and adverse events. In all, 274 patients (91.0%) received combination chemotherapy and 24 patients (8.0%) received monochemotherapy. During the study period, 53.8% of patients experienced adverse events ≥ G3. Haematologic and non-haematologic adverse events ≥ G3 occurred in 37.2% and 37.9% of patients, respectively. By completion of the first chemotherapy cycle, 19.9% of patients experienced adverse events ≥ G3 (12.0% haematologic and 12.0% non-haematologic adverse events). The most common haematologic adverse events ≥ G3 were neutropaenia (28.2%), anaemia (11.6%), thrombocytopaenia (8.3%), and febrile neutropaenia (4.3%). The most common non-haematologic adverse events ≥ G3 were fatigue (7.6%), anorexia (6.3%), abdominal pain (5.0%), nausea (4.7%), and diarrhoea (3.3%) (Table 3). G5 adverse events occurred in 14 patients (4.0%), which consisted of dyspnea (3 patients), sepsis (3 patients), febrile neutropaenia (1 patient), ileus (1 patient), lung infection (1 patient), multi-organ failure (1 patient), peritoneal infection (1 patient), pneumonitis (1 patient), thromboembolic event (1 patient), and supraventricular tachycardia (1 patient). In all, 6 of 14 G5 adverse events were considered to be related with treatment according to the investigator.
Predictive variables associated with occurrence of adverse events ≥ G3
Predictive variables were selected in the univariate analysis, which included clinical parameters such as age, sex, ECOG performance status, cancer type, chemotherapy-related variables, and each item from every domain in the GA. Six variables showed a significant association with incidence of adverse events ≥ G3. Six variables included serum protein < 6.7 g/dL, initial full-dose chemotherapy, suffering from psychological stress or acute disease in the past 3 months, water consumption of less than three cups per day, not being able to obey command of “take a piece of paper in your hand”, and self-perception of “not in good health” (Table 4).
Developing a prediction tool for occurrence of adverse events ≥ G3
Several different models were developed based on the results in the univariate/multivariate analyses. Those six variables that showed significance in the univariate analysis were included in the multivariate analysis, with four of them remaining significant in the all multivariate models: serum protein < 6.7 g/dL; initial full-dose chemotherapy; suffering from psychological stress or acute disease in the past 3 months; and water consumption of less than three cups per day. Model 1 consists of these four variables. Models 2 and 3 were developed by adding another variable to model 1 for up to five variables. Model 4 consisted of all variables that were significant in the univariate analysis. In each model, scores for each variable were assigned based on hazard ratio in the multivariate analysis (Table 5).
To select the best model for the prediction of adverse events ≥ G3 with the highest discriminatory ability, c-statistics were calculated. Model 4 showed the highest mean c-statistic (0.646, Supplementary Table 2). Finally, model 4, consisting of six variables, was confirmed as the most accurate prediction tool for occurrence of adverse events ≥ G3 (Supplementary table 3).
Prediction tool for occurrence of adverse events ≥ G3
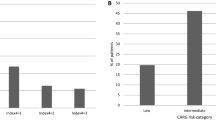
The predication tool indicated scores ranging 0–8 point. Patients with higher scores had more risk for adverse events ≥ G3. Score distribution is indicated in supplementary figure 1. Risk groups were classified according to score as low risk (0, 1), medium-low risk (2, 3), medium-high risk (4, 5), and high risk (6, 7, 8). Of patients classified by risk groups, 61 (21.0%), 143 (49.3%), 66 (22.8%), and 20 (6.9%) were in low, medium-low, medium-high, and high-risk groups, respectively. In each risk group, predicted cumulative incidence of adverse events ≥ G3 increased with the chemotherapy cycle number. In the low-risk group, predicted cumulative incidence of adverse events ≥ G3 was 9.9%, 19.2%, 26.1%, 32.8%, and 35.9% in cycle 1, cycle 2, cycle 3, cycle 4, and cycle 5, respectively. In the medium-low-risk group, predicted cumulative incidence of adverse events ≥ G3 was 16.0%, 30.1%, 39.8%, 48.7%, and 52.6% in cycle 1, cycle 2, cycle 3, cycle 4, and cycle 5, respectively. In the medium-high-risk group, predicted cumulative incidence of adverse events ≥ G3 was 24.4%, 43.6%, 55.6%, 65.5%, and 69.7% in cycle 1, cycle 2, cycle 3, cycle 4, and cycle 5, respectively. In the high-risk group, predicted cumulative incidence of adverse events ≥ G3 was 37.0%, 60.9%, 73.4%, 82.3%, and 85.5% in cycle 1, cycle 2, cycle 3, cycle 4, and cycle 5, respectively. In the same cycle, predicted cumulative incidence of adverse events ≥ G3 was also discriminated according to risk group (Fig. 1).
Discussion
From this prospective longitudinal multicentre study, we developed a novel prediction tool to identify those patients at risk for adverse events ≥ G3 in older patients undergoing first-line chemotherapy. This prediction tool consisted of six variables from clinical parameters and GA conducted pre-chemotherapy, with an ability to identify four risk groups (low, medium-low, medium-high, and high), which quantified cumulative risk rates of adverse events ≥ G3 from the first to the fifth cycle.
There are two established and validated prediction tools for severe chemotherapy toxicity (G4 haematologic toxicity and G3/4 non-haematologic toxicity; G3-5 chemotherapy-related toxicity).10,11,12 Nevertheless, our prediction tool has distinct features in terms of study population, primary outcome, and modeling methods as compared to previous studies.
This study population consisted of Koreans and patients initiating first-line chemotherapy. Previous studies were conducted in hospitals of the United States with most patients of Caucasian race. Toxicity profiles from chemotherapy are reported to be different by geographic regions due to ethnic differences in drug metabolism, nutritional status, social support, and medical culture.19,30 For example, Asians showed more frequent occurrence of febrile neutropaenia (18.6% vs 7.1%), edema (26.1% vs 5.4%), myalgia (42.3% vs 14.7%), and decreased appetite (47.0% vs 19.1%) in the phase III CLEOPATRA trial.30
Moreover, our study population showed a lower median BMI (22.5 vs. 25.9 in a previous study conducted in the United States),10 included more gastrointestinal cancer types, more frequent dose reductions (58.8% vs 24.0%) as compared with previous study. The different population characteristics and tolerances to chemotherapy justify the development chemotherapy prediction tools developed in Asian populations. Furthermore, patients in this study who were initiating only first-line chemotherapy were enrolled in contrast with previous studies, which allowed prior palliative chemotherapy. Vulnerability for chemotherapy could be different across chemotherapy lines. With additional lines of chemotherapy, chemotherapy toxicity may occur more frequently. It would be more ideal to include homogenous populations in terms of number of chemotherapy lines to develop precise chemotherapy toxicity prediction tool.
Regarding the primary outcome, in contrast with other studies using treatment-related toxicity, our study used adverse event as the primary measure regarding outcome. It is often difficult to determine the causality of adverse events in clinical practice. In view of older patients with cancer and their family members, events itself during chemotherapy are important regardless of causality. Therefore, adverse event is a suitable outcome measure in this study with older patients with cancer.
In terms of modeling methods, our prediction models were developed using the Cox proportional-hazards model, in which applied chemotherapy cycles and cycle with first adverse event ≥ G3 were incorporated. Therefore, cumulative incidence of adverse event ≥ G3 could be suggested on the contrary to generating just dichotomous outcomes in previous studies. This point is important because incidence of adverse events increase inevitably with proceeding of chemotherapy cycles and most patients recover from adverse event ≥ G3 and continue the next cycle. In our study, 45% of patients in the high-risk group were expected to experience adverse events ≥ G3 during the first cycle. However, almost all patients in the high-risk group were expected to experience adverse events ≥ G3 within fourth cycle. Meanwhile, no more than 30% of patients in the low-risk group were expected to have adverse events ≥ G3 as the cycles proceeded. This information might be valuable to decide and discuss chemotherapy application with older patient with cancer and their family members.
Finally, our prediction tool can utilise a questionnaire of only six questions, which would allow for a simple clinical application in busy oncology clinics. Six questions were related to nutritional status (two questions), recent illness (one question), chemotherapy dosing (one question), cognitive function (one question), and self-estimation for health status (one question). These components were reported as important factors in previous studies associated with geriatric outcome.6,10,12,21 In previous studies for the prediction of chemotherapy toxicity, chemotherapy dosing, nutritional status, and cognitive function were also included.10,12 Our prediction tool suggested cumulative incidence of adverse events ≥ G3 in each cycle of first-line chemotherapy with a mean c-statistic of 0.646 to predict adverse events ≥ G3, which is comparable to discriminative power found in previous studies.10,11,12
There are some limitations to this study. First, the applied chemotherapy regimens and cancer types were heterogeneous. It would be ideal to conduct this study in a specific tumour type, being treated with a specific chemotherapy regimen. However, our study aimed to determine common geriatric factors that affect the occurrence of adverse events ≥ G3. Compared with previous studies, only patients who would receive first-line chemotherapy were enrolled for a more homogenous study population. Second, G2 adverse events are also important in vulnerable older patients with cancer who are receiving chemotherapy. Hospitalisation, laboratory abnormality or symptoms etc. to stop chemotherapy, and mortality during chemotherapy could be a good outcome measure. However, these measures are mostly covered in adverse events ≥ G3, which were defined as the primary outcome in this study. Third, this study was performed in Korean patients and patients with first-line chemotherapy, but external validation in different populations or other Asian countries should be conducted. Fourth, in this study population, the risk scores of previous tools could not be calculated due to discrepancy in domains used in GA across studies. Direct comparison of efficacy of this tool with previous tools could not be performed. This prediction tool was designed in different population from previous studies, such as clinically homogeneous and Asian population. Furthermore, cumulative incidence showed in our prediction tool could give another information to clinic practice. Therefore, it would be worth to develop this tool regardless of comparison of efficacy with previous tools.
We developed simple, six-item novel prediction tool for adverse events ≥ G3, which would be easier to use in daily practice and which could provide patients and physicians information to plan chemotherapy in an Asian population. In high-risk patients, a high incidence of adverse events should be anticipated, and preventive and proactive measures should be administered. In other hands, active chemotherapy could be encouraged for patients in the low-risk group. Future studies are needed to evaluate geriatric intervention in high-risk patients to promote the safe use of chemotherapy in older patients with cancer.
References
Muss, H. B. et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. J. Clin. Oncol. 25, 3699–3704 (2007).
Hurria, A. et al. Role of age and health in treatment recommendations for older adults with breast cancer: the perspective of oncologists and primary care providers. J. Clin. Oncol. 26, 5386–5392 (2008).
Autier, P. Age at cancer diagnosis and interpretation of survival statistics. Lancet Oncol. 17, 847–848 (2016).
Kornblith, A. B. et al. Survey of oncologists’ perceptions of barriers to accrual of older patients with breast carcinoma to clinical trials. Cancer 95, 989–996 (2002).
Jung, H. W. et al. Multidimensional Geriatric Prognostic Index, based on a geriatric assessment, for long-term survival in older adults in Korea. PLoS ONE 11, e0147032 (2016).
Kim, J. W. et al. The early discontinuation of palliative chemotherapy in older patients with cancer. Support. Care Cancer 22, 773–781 (2014).
Kim, Y. J. et al. Comprehensive geriatric assessment in Korean elderly cancer patients receiving chemotherapy. J. Cancer Res. Clin. Oncol. 137, 839–847 (2011).
Kim, S. W. et al. Multidimensional frailty score for the prediction of postoperative mortality risk. JAMA Surg. 149, 633–640 (2014).
Soubeyran, P. et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J. Clin. Oncol. 30, 1829–1834 (2012).
Extermann, M. et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 118, 3377–3386 (2012).
Hurria, A. et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J. Clin. Oncol. 34, 2366–2371 (2016).
Hurria, A. et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J. Clin. Oncol. 29, 3457–3465 (2011).
Kagawa-Singer, M., Dadia, A. V., Yu, M. C. & Surbone, A. Cancer, culture, and health disparities: time to chart a new course? Ca Cancer J. Clin. 60, 12–39 (2010).
Lannin, D. R. et al. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA 279, 1801–1807 (1998).
Shim, E. J. et al. Health-related quality of life in breast cancer: a cross-cultural survey of German, Japanese, and South Korean patients. Breast Cancer Res. Treat. 99, 341–350 (2006).
Wellisch, D. et al. An exploratory study of social support: a cross-cultural comparison of Chinese-, Japanese-, and Anglo-American breast cancer patients. Psychooncology 8, 207–219 (1999).
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–386 (2015).
O’Donnell, P. H. & Dolan, M. E. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin. Cancer Res. 15, 4806–4814 (2009).
Hasegawa, Y. et al. Ethnic Difference in Hematological Toxicity in Patients with Non-small Cell Lung Cancer Treated with Chemotherapy A Pooled Analysis on Asian versus Non-Asian in Phase II and III Clinical Trials. J. Thorac. Oncol. 6, 1881–1888 (2011).
Wildiers, H. et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J. Clin. Oncol. 32, 2595–2603 (2014).
Kim, J. W. et al. A novel geriatric screening tool in older patients with cancer: the Korean Cancer Study Group Geriatric Score (KG)-7. PLoS ONE 10, e0138304 (2015).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40, 373–383 (1987).
Mahoney, F. I. & Barthel, D. W. Functional evaluation: the Barthel Index. Md. State Med. J. 14, 61–65 (1965).
Won, C. W., Rho, Y. G., Duk, S. W. & Lee, Y. S. The validity and reliability of Korean Instrumental Activities of Daily Living (K-IADL) scale. J. Korean Geriatr. Soc. 6, 273–280 (2002).
Won, C. W. et al. The development of Korean Activities of Daily Living (K-ADL) and Korean Instrumental Activities of Daily Living (K-IADL) scale. J. Korean Geriatr. Soc. 6, 107–120 (2002).
Podsiadlo, D. & Richardson, S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 39, 142–148 (1991).
Lee, D. Y. et al. The prevalence of dementia in older people in an urban population of Korea: the Seoul study. J. Am. Geriatr. Soc. 50, 1233–1239 (2002).
Bae, J. N. & Cho, M. J. Development of the Korean version of the Geriatric Depression Scale and its short form among elderly psychiatric patients. J. Psychosom. Res. 57, 297–305 (2004).
Vellas, B. et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 15, 116–122 (1999).
Swain, S. M. et al. Safety profile of pertuzumab with trastuzumab and docetaxel in patients from Asia with human epidermal growth factor receptor 2-positive metastatic breast cancer: results from the phase III trial CLEOPATRA. Oncologist 19, 693–701 (2014).
Acknowledgements
We are grateful to Hyun Jung Yoo R.N., Si Young Park R.N. (geriatric nurses in Seoul National University Bundang Hospital), and Prof. Myung Sook Park (Department of Nursing, Konkuk University, Chungju, Korea) who helped with geriatric assessment workshops, and Keum Sung Lim (data manager) and Mi Jin Yang (clinical research associate in KCSG data center) who helped to collect and manage data.
Funding
This study was supported by grants from the National R&D Program for Cancer Control, Ministry for Health and Welfare, Republic of Korea (study no: 1320370).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Note: This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International licence (CC BY 4.0).
Electronic supplementary material
Rights and permissions
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kim, J.W., Lee, YG., Hwang, I.G. et al. Predicting cumulative incidence of adverse events in older patients with cancer undergoing first-line palliative chemotherapy: Korean Cancer Study Group (KCSG) multicentre prospective study. Br J Cancer 118, 1169–1175 (2018). https://doi.org/10.1038/s41416-018-0037-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-018-0037-6
This article is cited by
-
Relationship between markers of malnutrition and clinical outcomes in older adults with cancer: systematic review, narrative synthesis and meta-analysis
European Journal of Clinical Nutrition (2020)
-
Management of the toxicity of chemotherapy and targeted therapies in elderly cancer patients
Clinical and Translational Oncology (2020)