Abstract
Objective This exploratory post hoc analysis sought to investigate clinical outcomes comparing non-surgical treatment for periodontal disease using exclusively hand instruments, exclusively ultrasonic instruments or a combination approach. Differences in time efficiency and equipment use with each treatment method were evaluated.
Methods In total, 55 patients with periodontitis were treated across two studies (randomised controlled trial and cohort study) with non-surgical periodontal therapy using hand instruments (HI), ultrasonic instruments (UI) or a combination approach (CI). All patients were re-evaluated 90 days after treatment. Clinical parameters, time taken and financial implications of non-surgical periodontal therapy were explored with a descriptive analysis within this post hoc analysis.
Results There were no clinically relevant differences in clinical parameters across all groups at day 90. Inter-group comparisons showed no clinically relevant differences in treatment outcome between groups. UI required less time on average to complete treatment compared to HI. UI provided using a half mouth approach had fewest overall episodes of expenditure and lowest maintenance costs.
Conclusions Comparison of clinical outcomes between HI, UI and CI yielded no clinically relevant differences. When comparing HI and UI, UI had a shorter treatment time on average. Full mouth treatment was associated with the least patient visits. UI was least costly on a recurring basis.
Key points
-
Clinical outcomes of non-surgical treatment for generalised periodontal disease demonstrate no clinically relevant differences across exclusively hand instrumentation, exclusively ultrasonic instrumentation and a combination of these techniques.
-
Selection of instrumentation technique for periodontal treatment may be dictated by factors other than clinical outcome.
-
Ultrasonic instrumentation provided by full mouth debridement within 24 hours yields similar clinical outcomes to 'quadrant' treatment with a combination of instruments. Full mouth debridement with ultrasonic instruments may offer the benefit of reducing clinic time and reducing costs of instrument sterilisation, reprocessing and maintenance.
Similar content being viewed by others
Introduction
Periodontal disease is one of the most common chronic diseases and severe periodontal disease affects approximately 10% of adults worldwide.1,2 The mainstay of treatment of periodontal disease is non-surgical therapy.
Initial 'step one' treatment includes removal of supragingival deposit and plaque retention factors and engagement of the patient in maintaining good oral hygiene.3 The second step of treatment involves subgingival professional mechanical plaque removal (PMPR). The two principal methods of subgingival PMPR are hand instrumentation and ultrasonic instrumentation. Hand instrumentation uses a range of specially designed curettes to remove deposits from root and tooth surfaces. Ultrasonic instrumentation is a subset of 'powered instrumentation techniques' and a range of inserts are available to remove plaque and calculus from the root surface using a rapidly vibrating metallic tip connected to a water irrigation system, which keeps the tip cool and flushes debris from the operating site. The ultimate goal of both techniques is to remove biofilm, plaque and calculus.
Periodontal instrumentation techniques have been compared through a variety of measures. Studies have demonstrated equal efficacy in probing depth reduction, clinical attachment gain,4,5 bleeding on probing (BOP) reduction,6,7 plaque removal ability8 and reduction of red complex bacteria.9 Currently, the choice of which instrumentation technique to utilise rests, in general, within the clinician's personal preference. In clinical practice, it is common for an operator to combine instrumentation techniques during a course of non-surgical periodontal therapy, thus providing a 'combination or blended approach' to treatment.
Regardless of the chosen instrumentation technique, the scheduling of treatment delivery also warrants consideration. The clinician has the option of providing root surface instrumentation on a quadrant-by-quadrant basis or by a full mouth approach. Quadrant instrumentation and full mouth approach are generally provided over four and one or two appointments, respectively, and evidence suggests comparable clinical outcomes.10 Operator preference and patient availability for treatment are currently key factors in decision-making for choice of treatment delivery approach. For some patients with cardiovascular disease, there may be a preference to deliver treatment in visits of no more than 30-45 minutes to minimise the systemic impact of periodontal treatment.11 The COVID-19 pandemic and the complication of aerosol generating procedures (AGPs) created another factor to consider in instrumentation technique choice.12,13
The importance of providing safe, efficient, timely and patient-centred care is paramount to quality within healthcare.14 Evidence would suggest that non-surgical periodontal therapy provided by hand or ultrasonic instrumentation fulfils these goals.15 Cost effectiveness in periodontitis treatment delivery has been explored in the literature within a variety of settings: a private practice in the USA,16 a public sector specialist practice in Malaysia17 and a case report within the UK of implications on diabetic management.18 As a result of these studies and others exploring supportive periodontal care, the perceived value of non-surgical periodontal treatment is high. Analysis of the cost of periodontal treatment within secondary care centres in the UK is worthy of consideration,19 particularly in the context of such a prevalent disease as periodontitis.
This post hoc, retrospective, analytical study aims to explore the clinical outcomes of periodontal treatment, comparing ultrasonic instrumentation, hand instrumentation and a combination approach, and comparing treatment delivery approaches (quadrant versus full mouth). An explorative cost-minimisation analysis of periodontal therapy will be presented. In addition, the study aimed to explore financial aspects of delivering periodontal therapy within an NHS dental hospital.
Methods and materials
This study is a post hoc analysis using pooled data generated by two separate studies: a randomised controlled trial (RCT) and a cohort study.20,21 Both studies had the primary outcome of investigating systemic inflammatory effects following non-surgical periodontal treatment. The current study evaluated secondary outcome data from these studies and analysed the clinical effects of non-surgical periodontal therapy by one of three approaches: hand instrumentation (HI), ultrasonic instrumentation (UI) and combination instrumentation (CI).
Patients referred to Glasgow Dental Hospital for periodontal care were recruited to each study using identical inclusion and exclusion criteria.
Criteria for inclusion in the study included: male or female patients aged between 18-70 years old and the presence of probing depths ≥5 mm on two or more teeth at non-adjacent sites with cumulative probing depths of ≥40 mm. Cumulative probing depth was calculated by examining six sites on each tooth. The deepest site on each tooth was recorded and if the value was >4 mm, this contributed to the cumulative total, with each tooth being only counted once towards the total.
Study exclusion criteria included: suspected or high risk for tuberculosis, hepatitis B or human immunodeficiency virus infections; requiring the services of an interpreter to understand and provide consent or any other reasons for inability to provide written, informed consent; presence of systemic illness, including bleeding diathesis, cardiovascular, liver, renal, or any regular medication requirements to control systemic disease; those pregnant or lactating; any treatment of a pharmacological nature within one month before study commencement, including routine use of any over-the-counter medications; and any specialist periodontal treatment in the preceding six months.
To provide a matched analysis, the two groups from the RCT (which were block randomised to ensure similar numbers in each group) (n = 19 in HI; n = 18 in UI) were matched with n = 18 patients in the cohort study. Patients were matched according to cumulative probing depths and age.
Clinical data (periodontally inflamed surface area [PISA], periodontal pocket depth [PPD], total pockets ≥5 mm%, clinical attachment loss [CAL], BOP% and plaque%) were extracted from datasets of both studies and combined for statistical analysis. Guidelines in the declaration of Helsinki were followed throughout. PISA is calculated using a seven-step process and involves reference to root surface area, CAL, BOP and recession measurements. PISA is calculated for each tooth in turn and then combined to provide an overall value for inflamed surface area for the whole mouth.22
To explore the implications of non-surgical periodontal treatment, data relating to procurement, processing and maintenance cost of interventions performed within the current study was gathered. An exploratory cost minimisation analysis was carried out; this tool allows comparison of the relative costs per course of treatment when alternative therapies have similar clinical outcomes. Reference is made to the treatment delivery approach (full mouth in 24 hours [completed in two visits] or quadrant by quadrant [usually completed in four visits - in this group, completed in 4.3 visits on average]). Depending on treatment group, a selection of instruments (either hand or ultrasonic) were used to achieve comprehensive treatment (Fig. 1).
Instruments used within current study treatment groups. a) Periodontal instruments used in treatment groups: exclusively ultrasonic instruments (left to right: Cavitron Slimline 10S 30K, Cavitron Slimline 10L 30K, Cavitron Slimline 10R 30K, Cavitron Thinsert 30K, Cavitron Powerline 1000 30K; Dentsply Sirona). b) Exclusively hand instruments and a combination of the above (left to right: Gracey 1/2, Gracey 7/8, Gracey 11/12, Gracey 13/14, Columbia 4L-4R, Hoe Scaler-lateral, Hoe Scaler-posterior; LM Dental)
All patients underwent baseline examinations, including plaque and gingivitis indices, full six-point periodontal pocket charting and standardised oral hygiene instruction before non-surgical periodontal therapy (Fig. 2). All treatment and clinical data collection was carried out by an experienced dental hygienist or specialist trainee in restorative dentistry. Both operators completed pocket charts on the first 12 patients in the RCT, demonstrating agreement (inter-examiner kappa score 0.66, using PPD as the assessment variable).
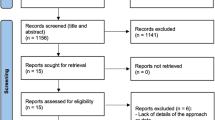
Study flowchart. Patients were recruited from new patient periodontology clinics within Glasgow Dental Hospital. Following eligibility assessment and consent processes, patients within the RCT underwent randomisation. An identical baseline/step one visit was carried in both studies. Note, the studies did not take place in parallel. The RCT was active between February 2018 and June 2019 and the cohort trial was active from August 2017 and September 2018
Instrumentation in the 'combination instrumentation' group received treatment on a quadrant-by-quadrant basis. Instrumentation in the 'hand instrumentation' and the 'ultrasonic instrumentation' arms received treatment using a full mouth approach completed within 24 hours.
Study data were entered into SPSS Statistics (v26, IBM Corp) using anonymous patient codes and analysed. Graphics were produced using PRISM (v8, GraphPad). All outcome data were summarised using mean (standard deviations [SDs]) or median (Q1, Q3), depending on their distribution. Non-symmetrical data were ln-transformed. Within groups, differences were tested using Wilcoxon signed-rank testing. General linear models (GLMs) were utilised to test differences in clinical variables (ln-transformed as required) between groups after adjusting for baseline level of variable of interest, number of teeth, smoking status, age and sex at day 90 follow-up. Treatment time comparison between HI and UI was carried out using a GLM adjusting for number of teeth and baseline disease severity (as measured by PISA). As a further subgroup analysis, correlation between treatment time and disease severity (PISA mm2) was investigated using Pearson's correlation coefficient.
Results
Data from a total of 55 patients across two studies were analysed.
Baseline characteristics were generally similar across groups; however, more smokers and fewer women were present in the CI group (Table 1). This was due to lack of available data in the CI group, following matching for disease severity and age.
Following treatment, there was significant improvement in clinical parameters (p <0.001 for all clinical parameters comparing pre-treatment and post-treatment timepoints), regardless of the treatment group (Fig. 3, Table 2). The observed pre- versus post-treatment changes were deemed clinically expected, with clinical changes in agreement with recently published literature of anticipated outcomes from non-surgical periodontal treatment.15,23
Clinical outcomes of periodontal treatment. Patients were treated with exclusively hand instruments (grey bars, n = 19), exclusively ultrasonic instruments (dotted bars, n = 18), or a combination of both instruments (white bars, n = 18). PISA was recorded before and 90 days after treatment. (Note: *** = p <0.001 comparing pre and post treatment within each group by Wilcoxon signed-rank test for related samples. Data are presented as Tukey boxplots (horizontal bar = median, + = mean, whiskers = minimum and maximum, circles = outliers as separate data points). a) PISA; b) PPD; c) total pockets more than or equal to 5 mm; d) CAL; e) BOP; f) plaque
All groups demonstrated improved clinical parameters (PISA, PPD, BOP, plaque%, pockets, CAL), with equal improvements in all groups at follow-up (adjusting for baseline levels of clinical variable, smoking status, treatment time, age, sex and number of teeth) (Table 2).
Time for treatment completion was measured using a stopwatch from the initial contact of a periodontal instrument on a tooth/root surface until treatment was deemed complete by the operator. Precise data of time of instrumentation were available only for hand and ultrasonic groups. Due to limitations in data availability, comparable data were not available for the combination treatment group. Mean (SD) for total treatment time for HI was 96.9 (23.08) minutes compared to 75.39 (17.83) minutes for UI. A GLM was employed for testing inter-group differences in treatment time, controlling for baseline disease severity (PISA at baseline) (p = 0.003; mean difference 21.51; 95% CI = 7.83-35.19). There was a weak positive correlation between baseline disease severity (as measured by baseline PISA and separately by baseline pockets ≥5 mm) and treatment time for UI (Pearson r = 0.33; Pearson r = 0.271, respectively). However, a greater positive correlation was found for treatment time using HI (Pearson r = 0.62; Pearson r = 0.76, respectively). This finding must be interpreted with caution, as the current study was not appropriately powered or designed to investigate such interactions.
Financial implications of periodontal treatment
Figure 4 demonstrates the number of events of expenditure associated with periodontal treatment, provided through either a single instrumentation approach or a combination approach to periodontal treatment.
CI was associated with the highest cost overall at the procurement stage due to the purchase of both ultrasonic and hand instruments (Fig. 4). If periodontal treatment was provided using a full mouth approach rather than a quadrant approach, the cost is reduced, due to fewer reprocessing cycles. Utilising a full mouth approach also resulted in a mean reduction of 1.3 patient visits to the clinic for treatment. Regarding maintenance, UI are recommended to be checked for wear of the tip before each use, taking approximately two minutes to evaluate five inserts and with no cost implications. The longevity of ultrasonic instruments is variable and must be closely monitored for clinical effectiveness. Maintenance of HI entails regular sharpening, ideally before each use. This incurs a further time burden and requires a particular skill set. Hand instruments also have a finite lifespan and can only be sharpened a given number of times until the instrument anatomy is ultimately compromised. Personal protective equipment (PPE) costs of ultrasonic treatment, due to the generation of aerosol, at the time of writing, were shown to be higher than hand instruments.
Discussion
To the best of our knowledge, this is the first descriptor of both clinical outcomes combined with exploration of financial implications associated with different periodontal treatment approaches in a UK setting. Periodontal disease is common, has a marked impact on patients and there is a recognised need for better access to effective treatment.24 To have any chance of achieving this within the healthcare system, the most efficient approaches to treatment will be required. A myriad of previous studies have compared HI and UI, but few have included any comparison with a 'combination' approach - the latter being the most commonly used in clinical practice. Our data are commensurate with previous work and show that periodontal parameters (BOP, plaque%, no pockets ³4 mm, CAL) demonstrated significant reductions following all instrumentation techniques provided.25,26,27 A full and thorough examination of the data from this study is explored within a 2021 Masters by Research thesis.28
A recent systematic review evaluating efficacy of UI compared to HI15 analysed six RCTs and reported no significant clinical differences following the use of ultrasonic and manual instrumentation techniques and no difference in frequency of adverse events. Although small in size, this finding is reflected in the current data, with no adverse events reported in either of our studies.
With regard to treatment delivery approach, clinical outcomes were similar when comparing full mouth instrumentation and quadrant-by-quadrant instrumentation. Each technique demonstrated similar clinical outcomes, in agreement with a recent systematic review.10
Our data show ultrasonic instrumentation, on average, was 21.51 minutes (95% CI: 9.22-34.62) faster than hand instruments to treat periodontal disease, in agreement with a contemporary systematic review23 and historical literature.6,29,30,31,32 Interestingly, treatment time for HI demonstrated a stronger correlation with disease severity, while ultrasonic showed a weaker correlation.20 It could therefore be speculated that UI offers greater time saving, particularly for the treatment of patients affected by more severe periodontal disease. Limitations of such a subgroup analysis must be considered when interpreting such speculation.
The COVID-19 pandemic has caused enormous disruption to dental services. There was a rapid realisation that pre-COVID measures for AGPs were insufficient in the face of a novel virus to which there was no population immunity. This resulted in changes to infection prevention and control measures for AGPs and the long-term impact remains to be seen. Nonetheless, at the time of writing, the need for additional measures remains in Scotland and seems unlikely to change in the near future. The long-term impact of the COVID-19 pandemic on dental infection prevention control measures remains to be seen. Therefore, the 'COVID-19' mitigation measures have been briefly considered here.12,33 Depending on the air changes per hour, the required fallow time may actually be accounted for in the time differential between hand and ultrasonic instruments, thus achieving treatment in the same time as HI, arguably with a less fatigued operator. Considering these data, notwithstanding aerosol PPE costs and discomfort, clinician preference may still be a deciding factor in instrumentation choice for periodontal treatment.
Full mouth treatment using a single modality was shown to incur fewer episodes of expense, per course of periodontal treatment (Fig. 4). This method of providing periodontal treatment was shown to result in fewer patient visits to the clinic for treatment, a reduction in overall PPE use and fewer sterilisation and repackaging cycles. These factors have positive implications not only for reduced expenditure, irrespective of the healthcare setting, but also for sustainability of this approach of treatment. Patients will also appreciate fewer visits to the clinic, resulting in less time off work and a reduction in the need for travel with such associated expense. Indeed, this patient-centred factor was identified in wider work related to this study.28
There is currently a paucity of literature on guidelines for hand instrument sharpening frequency, with some manufacturers advocating sharpening before each use. Within the study institution, hand instruments are sharpened approximately every 20 cycles, by an external contractor. Ultrasonic instruments have the benefit of no requirement for regular sharpening. Both instruments will eventually reach an end of life and require replacement. Unfortunately, no data were available on the regularity of replacement hand instruments or ultrasonic instruments.
This study could be limited by potential for selection bias. The patients all volunteered to take part in research; thus, it is likely that compliant patients self-select for inclusion. However, recent guidelines would indicate that completion of 'step one' of periodontal treatment will ensure that reasonably compliant patients will receive subgingival PMPR, and thus the finding would hopefully still have some applicability.3 The patients in this study (both the RCT and cohort datasets) completed 'step one' in a single visit. It is likely that patients who have not already been referred for specialist treatment and volunteered for a study may require more than one visit to complete 'step one.'
Due to the nature of periodontal treatment, patients were unable to be blinded during the treatment process. This could lead to performance bias, as patients receiving certain treatments may alter their compliance or self-performed oral hygiene, thus affecting clinical outcomes. A further source of performance bias is that of the influence of multiple independent variables between treatment groups. Due to limitations in available data, the influence of the independent variables of instrumentation technique and treatment delivery approach could not be explored in isolation. The presence of unknown confounders (such as stress level, level of physical activity and unknown genetic factors) may also have affected study results. There was potential for observation bias as the operator was - unavoidably - aware of which intervention was received by each patient. However, these limitations are inherent within interventional periodontal research. Interpretation of the statistical analyses in the current study warrant caution, as the data were obtained as secondary outcome variables from studies powered to detect changes in inflammatory mediators. These studies were not powered to detect differences in treatment time nor equivalence of clinical variables.
Treatment protocols presented in this study were based in a secondary care dental hospital setting. This may have some implications for the external validity of the findings. However, presentation of comparative cost implications of treatment approaches (Fig. 4) will have application in clinical practice, irrespective of the healthcare setting.
This study suggests the clinical outcome of non-surgical periodontal instrumentation techniques is similar, regardless of instrumentation technique and approach to treatment. There appeared to be cost and time saving with the use of single modality treatment using a full mouth approach using ultrasonic instruments. However, this treatment modality may not be suitable for all patients and all operators.
Conclusions
Within the limitations of this study, the following statements can be considered:
-
Well-performed, non-surgical periodontal treatment for generalised periodontitis results in predictable improvements in clinical measures of disease state, regardless of instrumentation technique
-
The use of UI results in a significant reduction in treatment time with a comparable clinical outcome, compared to HI
-
Full mouth instrumentation, particularly with ultrasonic instruments, was associated with fewer episodes of expenditure and fewer patient visits to the clinic than quadrant instrumentation.
These findings could be used to help improve efficiency, accessibility and sustainability of periodontal treatment.
References
Petersen P E, Ogawa H. Strengthening the prevention of periodontal disease: the WHO approach. J Periodontol 2005; 76: 2187-2193.
Bernabe E, Marcenes W, Hernandez C R et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J Dent Res 2020; 99: 362-373.
West N, Chapple I, Claydon N et al. BSP implementation of European S3 - level evidence-based treatment guidelines for stage I-III periodontitis in UK clinical practice. J Dent 2021; DOI: 10.1016/j.jdent.2020.103562.
Obeid P R, D'Hoore W, Bercy P. Comparative clinical responses related to the use of various periodontal instrumentation. J Clin Periodontol 2004; 31: 193-199.
Krishna R, De Stefano J A. Ultrasonic vs. hand instrumentation in periodontal therapy: clinical outcomes. Periodontol 2000 2016; 71: 113-127.
Badersten A, Nilvéus R, Egelberg J. Effect of nonsurgical periodontal therapy. I. Moderately advanced periodontitis. J Clin Periodontol 1981; 8: 57-72.
Oosterwaal P J, Matee M I, Mikx F H, van't Hof M A, Renggli H H. The effect of subgingival debridement with hand and ultrasonic instruments on the subgingival microflora. J Clin Periodontol 1987; 14: 528-533.
Thornton S, Garnick J. Comparison of ultrasonic to hand instruments in the removal of subgingival plaque. J Periodontol 1982; 53: 35-37.
Ioannou I, Dimitriadis N, Papadimitriou K, Sakellari D, Vouros I, Konstantinidis A. Hand instrumentation versus ultrasonic debridement in the treatment of chronic periodontitis: a randomized clinical and microbiological trial. J Clin Periodontol 2009; 36: 132-141.
Eberhard J, Jepsen S, Jervøe-Storm P-M, Needleman I, Worthington H V. Full-mouth treatment modalities (within 24 hours) for chronic periodontitis in adults. Cochrane Database Syst Rev 2015; DOI: 10.1002/14651858.CD004622.pub3.
Sanz M, Marco Del Castillo A, Jepsen S et al. Periodontitis and cardiovascular diseases: Consensus report. J Clin Periodontol 2020; 47: 268-288.
Ge Z, Yang L-M, Xia J-J, Fu X-H, Zhang Y-Z. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J Zhejiang Univ Sci B 2020; 21: 361-368.
Scottish Dental Clinical Effectiveness Programme. Mitigation of Aerosol Generating Procedures in Dentistry: A Rapid Review. 2021. Available at https://www.sdcep.org.uk/media/dnoart3g/sdcep-mitigation-of-agps-in-dentistry-rapid-review-v1-2-april-2021.pdf (accessed December 2022).
Institute of Medicine, Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington DC: National Academy Press, 2001.
Suvan J, Leira Y, Moreno Sancho F M, Graziani F, Derks J, Tomasi C. Subgingival Instrumentation for Treatment of Periodontitis. a systematic review. J Clin Periodontol 2019; DOI: 10.1111/jcpe.13245.
Bowen D M. Is non-surgical periodontal therapy cost effective? J Dent Hyg 2015; 89: 6-10.
Mohd-Dom T, Ayob R, Mohd-Nur A et al. Cost analysis of periodontitis management in public sector specialist dental clinics. BMC Oral Health 2014; 14: 56.
Solowiej-Wedderburn J, Ide M, Pennington M. Cost-effectiveness of non-surgical periodontal therapy for patients with type 2 diabetes in the UK. J Clin Periodontol 2017; 44: 700-707.
Iacobucci G. NHS long term plan: Care to be shifted away from hospitals in '21st century' service model. BMJ 2019; DOI: 10.1136/bmj.l85.
Johnston W, Paterson M, Piela K et al. The systemic inflammatory response following hand instrumentation vs ultrasonic instrumentation - a randomised controlled trial. J Clin Periodontol 2020; 47: 1087-1097.
Davison E, Johnston W, Piela K et al. The Subgingival Plaque Microbiome, Systemic Antibodies Against Bacteria and Citrullinated Proteins Following Periodontal Therapy. Pathogens 2021; 10: 193.
Nesse W, Abbas F, van der Ploeg I, Spijkervet F K L, Dijkstra P U, Vissink A. Periodontal inflamed surface area: quantifying inflammatory burden. J Clin Periodontol 2008; 35: 668-673.
Tunkel J, Heinecke A, Flemmig T F. A systematic review of efficacy of machine-driven and manual subgingival debridement in the treatment of chronic periodontitis. J Clin Periodontol 2002; DOI: 10.1034/j.1600-051x.29.s3.4.x.
Public Health England. Delivering better oral health: an evidence-based toolkit for prevention. 2017. Available at https://www.whittington.nhs.uk/document.ashx?id=14127 (accessed December 2022).
Smiley C J, Tracy S L, Abt E et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc 2015; 146: 508-524.
Graziani F, Karapetsa D, Alonso B, Herrera D. Nonsurgical and surgical treatment of periodontitis: how many options for one disease? Periodontology 2000 2017; 75: 152-188.
Wennström J L, Tomasi C, Bertelle A, Dellasega E. Full-mouth ultrasonic debridement versus quadrant scaling and root planing as an initial approach in the treatment of chronic periodontitis. J Clin Periodontol 2005; 32: 851-859.
Paterson M. The effect of instrumentation technique on the outcome of treatment for periodontitis. Glasgow: University of Glasgow, 2021. MScR Thesis.
Dragoo M R. A clinical evaluation of hand and ultrasonic instruments on subgingival debridement. Part I. With unmodified and modified ultrasonic inserts. Int J Periodontics Restorative Dent 1992; 12: 310-323.
Laurell L. Periodontal healing after scaling and root planing with the Kavo Sonicflex and Titan-S sonic scalers. Swed Dent J 1990; 14: 171-177.
Yukna R A, Scott J B, Aichelmann-Reidy M E, LeBlanc D M, Mayer E T. Clinical evaluation of the speed and effectiveness of subgingival calculus removal on single-rooted teeth with diamond-coated ultrasonic tips. J Periodontol 1997; 68: 436-442.
Breininger D R, O'Leary T J, Blumenshine R V. Comparative effectiveness of ultrasonic and hand scaling for the removal of subgingival plaque and calculus. J Periodontol 1987; 58: 9-18.
College of General Dentistry. Implications of COVID-19 for the safe management of general dental practice A practical guide. 2020. Available at https://cgdent.uk/wp-content/uploads/2020/10/FGDP-CGDent-Implications-of-COVID-19-for-the-safe-management-of-general-dental-practice-2-October-2020-v2.pdf (accessed December 2022).
Acknowledgements
The authors would like to thank Debbie MacKenzie and Clare Brown for their valuable assistance during both included studies. We would also like to thank the staff of the CSSD department of Glasgow Dental Hospital and Marilyn Goulding of Dentsply Sirona for support during the randomised controlled trial study. Thank you to Krystyna Piela and Annabel Simpson for their support in data management.
The current work is based on work detailed within the first author's MSc(R) thesis, submitted to University of Glasgow.
Funding
The current study received no direct funding.
One of the included study datasets (RCT) was jointly funded by the University of Glasgow's PhD programme and Dentsply Sirona.
Author information
Authors and Affiliations
Contributions
Michael Paterson: manuscript preparation and treatment of patients in RCT study. William Johnston: manuscript proofreading and day-to-day running of included RCT study. Andrea Sherriff: supervision of statistical aspects of study (RCT), project supervision and proofreading. Shauna Culshaw: chief investigator of both included studies, overall project supervision, manuscript and proofreading.
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Ethical approval was granted by application to the Greater Glasgow and Clyde Health Board and Office for Research Ethics Committees Northern Ireland (ORECNI) for both studies which served to provide data for the current analysis. This study, being a post hoc analysis, required no separate ethical approval.
All patients of each respective included study datasets provided informed, written consent to participate in the studies and have their data used for research purposes.
Rights and permissions
About this article
Cite this article
Paterson, M., Johnston, W., Sherriff, A. et al. Periodontal instrumentation technique: an exploratory analysis of clinical outcomes and financial aspects. Br Dent J (2023). https://doi.org/10.1038/s41415-022-5405-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41415-022-5405-1