Abstract
'Necrotising periodontal diseases' is an umbrella term for necrotising gingivitis, necrotising periodontitis, necrotising stomatitis and noma. These rapidly destructive conditions are characterised by pain, interdental ulceration and gingival necrosis which, if left untreated, can result in osteonecrosis. Research indicates that patients with a history of alcohol misuse are at an increased risk of malnutrition, which negatively affects the immune response and predisposition to necrotising periodontal diseases. This article will discuss that osteonecrosis of the alveolar bone does not exclusively occur in association with antiresorptive medications, but can occur as a severe form of necrotising gingivitis. In this article, we will describe two cases to highlight the occurrence, presentation and management of necrotising periodontal diseases secondary to alcohol misuse.
Key points
-
Raises clinician awareness on the topic of necrotising periodontal diseases and describes diagnosis and treatment modalities.
-
Explores and explains the link between alcohol misuse, malnutrition and necrotising periodontal diseases.
-
Encourages dentists to liaise with healthcare professionals in the management of patients suffering from underlying comorbidities, highlighting the importance of holistic patient care.
Similar content being viewed by others
Introduction
Alcohol consumption has negative effects on every major body system.1 In 2017/18, over 586,000 people in the UK were classified as dependent drinkers.2 Patients suffering from alcohol dependency are likely to be malnourished and immunocompromised, predisposing them to developing necrotising periodontal diseases (NPDs). Severe necrotising gingivitis in immunocompromised individuals can lead to necrosis of the alveolar bone, resulting in necrotising stomatitis. If left untreated, necrotising stomatitis results in extensive osteonecrosis and, in some extreme cases, noma.
Necrotising periodontal diseases
During the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions, the classification of NPDs was updated from the 1999 classification:3 'necrotising ulcerative gingivitis' and 'necrotising ulcerative periodontitis' were changed to 'necrotising gingivitis' (NG) and 'necrotising periodontitis' (NP), respectively. A third, new, disease stage was added and termed 'necrotising stomatitis' (NS). NG can be diagnosed if necrosis is limited to the interdental papilla, presenting with the characteristic 'punched out' appearance.4 If the inflammatory process is accompanied by rapid alveolar bone loss, along with the formation of a pseudomembrane, lymphadenopathy and pyrexia, NG is considered to have progressed to NP. Once the condition spreads to involve the alveolar bone, resulting in osteonecrosis and bony sequestration, this is termed NS. NG, NP and NS are stages of the same condition.3,5 A proposed staging mechanism of NPDs has been suggested by Horning,5 originally modified from Pindborg6 and Uohara7 (Table 1).
As per the consensus report produced during the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions, NPDs are thought to occur in two specific categories of patients, with the risk of progression to more severe stages of NPDs varying dependent on underlying risk factors (Table 2).3
Although the widespread prevalence of NPDs is considered to be <1%, it varies depending on the patient group. Approximately 1% of patients with NG may present with necrosis extending to the alveolar bone, but this will be dependent on underlying risk factors.5 In extreme cases, NPDs can result in noma (cancrum oris), which leads to extensive destruction of the soft and hard tissues of the face, and may eventually be fatal.4,8,9,10 NPDs are rarely seen in developed countries. Noma almost exclusively occurs in developing countries, where malnutrition and immunocompromise are more widespread. Alcohol misuse is a predisposing factor for the development of NPDs, through its detrimental effect on nutrition due to the reduced host immune response and the direct harmful effects of alcohol on the bone.5
Case one
In 2015, a 46-year-old woman attended a drop-in dental clinic for drug and alcohol users. She complained of painful gums that prevented her from eating and drinking. Severe halitosis was also causing great concern. Medical history revealed bipolar disorder, fibromyalgia and alcoholism, with the patient regularly exceeding 120-140 units/week. Routine prescribed medications were sodium valproate, sertraline, co-codamol, vitamin B and thiamine. There was no history of bisphosphonates or chemotherapeutic agents (for example, monoclonal antibodies), and no history of head and neck radiotherapy. She was an irregular dental attender but was highly motivated regarding her oral hygiene, prioritising her oral health daily. On examination, generalised extensive gingival inflammation was noted with additional denuding of bone around teeth 31, 32, 33, 12, 13 and 36, 37. Due to the inflammation and tenderness present, no basic periodontal examination was undertaken. Additionally, at the sites of bony exposure, oral hygiene was poor; otherwise, her dentition was fairly maintained with minimal plaque noted. There was no pus exuding from the affected sites. The denuded areas were provisionally diagnosed as NG. The initial treatment plan included scaling and provision of antibiotics. However, the patient was unable to tolerate any scaling at this appointment, and due to her alcohol intake, metronidazole was an inappropriate antibiotic of choice. A prescription of 6% hydrogen peroxide mouthwash, 0.15% benzydamine hydrochloride spray (Difflam) and a regime of 500 mg amoxicillin three times a day for five days was provided. At a subsequent review, the patient reported no improvement in pain and felt her lower teeth had become more mobile than previously. On examination, teeth 31, 32, 33 were grade I mobile.
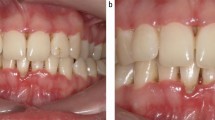
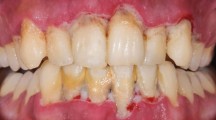
At this appointment, clinical photographs were taken (Figures 1, 2, 3 and 4).
An orthopantomogram (OPG) was taken (Fig. 5) which did not reveal any bony pathology nor bony sequestra. Diagnosis of generalised periodontitis stage III grade B with an associated risk factor of smoking was concluded. As a further management plan, the patient was encouraged to book an appointment with her general practitioner for blood tests, including HIV, hepatitis C and a full blood count, to eliminate any underlying immunocompromising condition. A referral was sent to the local oral and maxillofacial department for advice on management. Unfortunately, she did not attend her initial appointment due to transport difficulties and declined further referrals. A subsequent six-week further review revealed the patient complaining of intense pain localised to the lower incisal region. On examination, tooth 32 was grade III mobile and extracted using tweezers, with local anaesthetic deemed unnecessary. The supporting bone was found to be necrotic and avascular but non-mobile, and therefore was not removed. The patient was prescribed co-amoxiclav 250 mg/125 mg with an additional 250 mg amoxicillin three times a day for five days, and was advised to continue 6% hydrogen peroxide mouthwash with 0.2% chlorhexidine mouthwash prescribed in addition. Over the next six weeks, regular reviews were completed; oral pain had greatly reduced and the patient was able to eat soft food. However, she was concerned her remaining lower teeth were loose and 'felt like they didn't belong to her'. Due to increased mobility of teeth 31 and 33, these were extracted alongside sequestered mobile bone and partial closure was achieved with sutures. In line with guidance, no additional pre- or post-extraction precautions were required. She did not attend several booked review appointments; therefore, further examination was delayed for three months. The post-extraction site of the 31, 32, 33 region healed well, but the necrotic bone around the 12, 13, 14 region was extensive, with grade II mobility. These teeth were extracted alongside mobile bony sequestra. Six months after initial attendance, the patient successfully completed an alcohol detox programme. At review, although the patient had extensive gingival recession around teeth 22, 23 and 36, 37, following the sequestration of small bony fragments, the teeth were not mobile. Shortly after, the patient was able to begin periodontal therapy and had partial acrylic dentures constructed. She continues to experience dentine hypersensitivity due to extensive gingival recession, which is managed with high-fluoride toothpaste and desensitising toothpaste. Although clinically this patient was not seen by the local oral and maxillofacial department, following liaison with their team, it was proposed that she had developed osteonecrosis related to alcohol abuse. No post-treatment photographs are available.
Case two
A 53-year-old man presented with pain on his routine dental visit in June 2019. Tooth 45 had become increasingly painful and mobile but he did not report any swelling. He was a longstanding patient in the community dental service since 2008, with regular treatment visits for generalised periodontitis. Medical history revealed depression, bipolar disorder and alcohol dependency. He was drinking approximately 210-280 units/week for at least ten years. He has previously attempted detoxification with acamprosate but was unsuccessful. Routine prescribed medications included trazodone, zuclopenthixol, lansoprazole and amitriptyline alongside benzodiazepines (diazepam and temazepam) when required. He was allergic to mirtazapine and morphine patches. There was no history of bisphosphonate use, antiresorptive medications or chemotherapeutic agents (for example, monoclonal antibodies) nor previous radiotherapy.
At the first appointment, the patient presented with generalised truncation of the gingivae and a diagnosis of NG was given. A three-day course of 400 mg metronidazole three times a day was prescribed. The patient was made aware of the disulfiram-like interaction between alcohol and metronidazole, and agreed to abstain from drinking while taking the antibiotic and for 48 hours after completing the course. After seven days, a clinical review was completed. Full-mouth periodontal treatment was carried out for his generalised periodontitis, which highlighted a sequestrum of bone within the infrabony defect distal to tooth 45. This was non-mobile and subgingival. A periapical radiograph was taken (Fig. 6) which clearly indicated a bony sequestrum present distal to tooth 45. The tooth appears to have been previously restored with a fibre post, without a root canal treatment, and a widened periodontal membrane space was noted surrounding the apex. The radiograph was sent to an oral surgeon to discuss a provisional diagnosis and management options within the community clinic. This was assessed by a consultant in oral surgery and consultant radiologist, agreeing the likely diagnosis of osteonecrosis of the bone secondary to malnutrition due to alcohol misuse. Advice was given not to extract tooth 45 and to adopt a minimally invasive approach by allowing the area of exposed bone to exfoliate. Furthermore, 0.2% chlorhexidine mouthwash was prescribed, and with additional input from the microbiology team, 500 mg amoxicillin three times a day for six weeks was recommended. In the meantime, two further periodontal appointments were completed by the dental therapist in the following two months to prevent advancement of his generalised periodontitis.
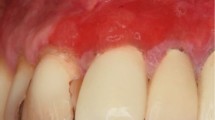
An appointment to review tooth 45 was arranged. Due to several unattended appointments, the review extended to October 2019 (four months after initial presentation). Improvement in oral hygiene was noted; however, the area of exposed bone was now supragingival extending to involve the cervico-enamel junction around tooth 45. The patient was advised this was likely to be osteonecrosis secondary to malnutrition due to alcohol misuse, but for definitive diagnosis and management, a referral to the oral surgery team would be required for specialist input. However, he declined this referral. Clinical photos and further radiographs were taken (Figures 7, 8 and 9) and the suggested management plan from oral surgery remained as before.
Diagnosis and a treatment plan were discussed with the patient to which he responded openly, accepting his alcohol misuse as the cause. However, after an unsuccessful previous detoxification attempt, this was not a feasible treatment option. He also declined prolonged use of antibiotics. Oral hygiene measures were reinforced and monthly reviews were agreed upon, with periodic radiographs and clinical photographs for vigilant monitoring. The risk of the area of exposed bone increasing in size was fully discussed as a risk of not accepting the ideal management plan.
On subsequent monthly reviews, no change in area of exposed bone was noted; no report of pain or swelling. Unfortunately, due to sporadic attendance, this patient did not attend the three subsequent appointments and no clinical review was carried out before COVID-19. Following telephone review, this patient did not report any pain and advised that his tooth and area of bone remained the same as before. Upon resuming dental services, this patient will be a high clinical priority for review.
Discussion
These patients presented with NP with exposed bone, which progressed to formation of bony sequestra, and thus the diagnosis of localised osteonecrosis and NS could be made. The necrosis seen occurred as a result of rapidly progressing NG, precipitated by immunocompromise and malnutrition secondary to alcohol misuse, and possibly due to the direct detrimental effects of alcohol on the bone and healing processes.11
Alcohol misuse and periodontal health
Despite a national downward trend in alcohol consumption, a high number of people consume excess alcohol.1 The link between alcohol misuse and periodontal disease is not well established; however, immunocompromise, malnutrition and poor oral hygiene are all predisposing factors and are closely interlinked. Higher levels of periodontal pathogens have been observed in patients who misuse alcohol, as well as higher levels of interleukin-1β and tumour necrosis factor alpha, which may all be implicated.12,13
Diagnosis of NPDs
While the clinical appearance of NPDs is distinctive, radiologically, the bone changes can be extensive (such as in case two) or minimal to non-existent (such as in case one). Osteomyelitis, medication-related osteonecrosis of the jaw and osteoradionecrosis, for instance, all result in moth-eaten, ragged, patchy radiolucent areas of the bone and can resemble osteonecrosis, but can be present without any radiological changes.14,15 In all these conditions, occasionally, radiopaque sequestra of necrotic bone may be visible.14 Computerised tomography (CT) scans are more reliable at differentiating between conditions of the bone and an OPG may be insufficient without clinical information.16 The same applies to histopathological diagnoses - studies have shown that without a clinical report, it is challenging to distinguish between the above conditions.17 Therefore, specific aspects of the clinical history will provide essential information when determining the final diagnosis.
Aetiology of NPDs
NPDs are thought to occur when the commensal microbiota within the oral cavity overrides the immune response, together with an infection with specific microorganisms such as P. intermedia, Treponema, Selenomonas and Fusobacterium species.9,18 Figure 10 demonstrates the pathogenesis in the development of bony necrosis in malnourished patients who are dependent on alcohol.
Pathogenesis of necrotising stomatitis as a result of alcohol misuse22
Step 1 (blue boxes)
Alcohol misuse is a predisposing factor to the development of NPDs, directly through negatively influencing the immune response and indirectly through an increased risk of malnutrition. Both of these can be seen in our patients and other similar case reports.5,19,20 The most influential risk factor has been found to be seropositivity to HIV infection.4,5 Poor oral hygiene increases the risk, but HIV-positive patients can present with these conditions even with good oral hygiene.5,9 Lack of sleep, stress and smoking have often been reported by patients presenting with NPDs and are also implicated.4,5,9
Step 2 (orange box)
Alcohol-dependent individuals are at an increased risk of malnutrition. Specific types of malnutrition have been implicated in the development of NPDs and are encountered in patients suffering from alcoholic hepatitis.21,22,23 The nature of the malnutrition in these patients can arise from calorie needs being met by alcohol consumption rather than a balanced diet, malabsorption, and the unbalanced metabolism of protein and nutrients due to liver damage.23
Step 3 (green boxes)
Malnourished patients are likely to suffer from deficiencies in vitamins A and C, zinc and albumin, which are specifically associated with NPDs, in addition to those which are classically linked to alcohol misuse (vitamins B1 [thiamine], B6, B12, C, K and folate).12,22 Furthermore, increased levels of cortisol and histamine, altered responses of immune cells (T cells, B cells, lymphocytes), altered cytokine response and altered levels of albumin are some of the resultant effects of malnutrition upon the immune system which predispose to NPDs.9,24
Step 4 (yellow boxes)
Impaired immune response predisposes to the overgrowth of specific bacteria.18 These bacteria directly contribute to the damage observed in NPDs by releasing endotoxins which damage periodontal tissues.4 They also cause indirect damage by stimulating the cells of the oral epithelium to release specific cytokines, which are implicated in the bone loss seen in NPDs by reducing the number of osteoblasts and increasing the number of osteoclasts.25
Step 5 (red box)
As NG is a noma precursor, the link between NG, noma and malnutrition has been extensively studied. It is a serious problem in developing countries. In studies by Enwonwu et al.,22 the authors found children suffering from noma are additionally affected by the vitamin, protein and hormone imbalances previously described. These factors negatively affect oral mucosal immunity and lead to the development of NG and subsequently noma.22,24 This highlights how reduced immunity results in the progression of NG to noma, which is possible in immunocompromised patients who misuse alcohol.
Treatment of NPDs and bony sequestra
Treatment of NPDs should be focused on the management of the underlying conditions. A HIV test should be conducted such as in case one and liaison with the general practitioner is indicated to rule out other immunocompromised states. If the patient can tolerate it, scaling should occur.4 If active open wounds are present on the gingivae, vigorous brushing should be avoided in the area and antimicrobial mouth rinses used instead (eg hydrogen peroxide or chlorhexidine).4 If the patient is immunocompromised, responds poorly to local measures or if there are signs of systemic involvement, systemic antibiotics may be prescribed. The first-line antibiotic is metronidazole 400 mg (three times a day for three days); however, if this is contraindicated, as is the case in patients who cannot abstain from alcohol, a three-day course of 500 mg amoxicillin three times a day can be provided.4,26 The sequestra will eventually become mobile and can be removed, often with no need for local anaesthesia.4 The necrotic bone may also exfoliate spontaneously.19 If necrosis of the alveolar bone is seen, referral to the oral and maxillofacial department is appropriate. Both of the patients described in this article declined referral for further investigations; therefore, holistic management was adopted using local measures and management of underlying risk factors. The treatment of extensive, spreading osteonecrosis of the jaw may require long-term antibiotics, surgical debridement of the necrotic tissue, or even resection and reconstruction of the jaw.27
Alcohol misuse and clinician responsibility
Clinicians must not forget the oral effects of excessive alcohol use, oral cancer being the greatest concern. Patients regularly consuming excessive alcohol are likely to be smokers, and in combination, these habits cause patients to be 38 times more likely to develop oral cancer.28 Alcoholic drinks are acidic in pH; therefore, tooth surface loss is likely. These individuals are at greater risk of dental and facial injuries, and in the long term are likely to develop mental health problems, such as depression and anxiety.12,29 Specific barriers to accessing and accepting dental care must also be considered30 (Table 3). The responsibility of the dental team in identifying and providing help to an alcohol-dependent patient must not be understated. The Delivering better oral health toolkit highlights the importance of a social history with use of specific screening questionnaire tests such as AUDIT-C.29 Public Health England have developed Alcohol Identification and Brief Advice to educate dental health professionals on the importance of early intervention advice regarding alcohol consumption.31
Conclusion
Alcohol misuse and its negative effects due to malnutrition on the human body have been extensively researched. The concern of malnutrition leading to NPDs is highlighted in this paper, alongside the harmful physiological and immunological effects. The prevalence of NPDs in patients suffering from alcohol dependency requires further study. Nevertheless, this paper emphasises the multiple oral effects of alcohol, the importance of vigilant regular dental examinations to prevent any detrimental consequences and the importance of the dental team in supporting such patients.
References
Public Health England. The Public Health Burden of Alcohol and the Effectiveness and Cost-Effectiveness of Alcohol Control Policies. London: Public Health England, 2016.
Public Health England. Public Health Profiles: Proportion of dependent drinkers not in treatment (%) (Current method). London: Public Health England, 2019.
Papapanou P N, Sanz M, Buduneli N et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018; 89 Suppl 1: S173-S182.
Lang N P, Lindhe J (eds). Clinical Periodontology and Implant Dentistry, 2 Volume Set. 6th ed. Chichester, John Wiley & Sons, 2015.
Horning G M, Cohen M E. Necrotizing Ulcerative Gingivitis, Periodontitis, and Stomatitis: Clinical Staging and Predisposing Factors. J Periodontol 1995; 66: 990-998.
Pindborg J J, Bhat M, Roed-Petersen B. Oral Changes in South Indian Children with Severe Protein Deficiency with Special Reference to Periodontal Conditions. J Periodontol 1967; 38: 218-221.
Uohara G I, Knapp M J. Oral fusospirochetosis and associated lesions. Oral Surg Oral Med Oral Pathol 1967; 24: 113-123.
Herrera D, Retamal-Valdes B, Alonso B, Feres M. Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J Periodontol 2018; 89: S85-S102.
Folayan M O. The epidemiology, aetiology, and pathophysiology of acute necrotizing ulcerative gingivitis associated with malnutrition. J Contemp Dent Pract 2004; 5: 28-41.
Almazrooa S A, Woo S-B. Bisphosphonate and nonbisphosphonate-associated osteonecrosis of the jaw. J Am Dent Assoc 2009; 140: 864-875.
Chang C, Gershwin M E. Alcohol-induced osteonecrosis. Crit Rev Phys Rehabil Med 2004; 16: 257-271.
Kwasnicki A, Longman L, Wilkinson G. The significance of alcohol misuse in the dental patient. Dent Update 2008; 35: 7-20.
Lages E J P, Costa F O, Cortelli S C et al. Alcohol Consumption and Periodontitis: Quantification of Periodontal Pathogens and Cytokines. J Periodontol 2015; 86: 1058-1068.
Whaites E, Drage N. Essentials of dental radiography and radiology. 5th ed. China: Churchill Livingstone, 2013.
Koong B. Atlas of Oral and Maxillofacial Radiology. 1st ed. Chichester: John Wiley & Sons, 2017.
Obinata K, Shirai S, Ito H et al. Image findings of bisphosphonate related osteonecrosis of jaws comparing with osteoradionecrosis. Dentomaxillofac Radiol 2017; 46: 20160281.
De Antoni C C, Matsumoto M A, da Silva A A et al. Medication-related osteonecrosis of the jaw, osteoradionecrosis, and osteomyelitis: A comparative histopathological study. Braz Oral Res 2018; DOI: 10.1590/1807-3107bor-2018.vol32.0023.
Loesche W J, Syed S A, Laughon B E, Stoll J. The Bacteriology of Acute Necrotizing Ulcerative Gingivitis. J Periodontol 1982; 53: 223-230.
Zushi Y, Noguchi K, Moridera K, Takaoka K, Kishimoto H. Osteonecrosis of the jaw in an AIDS patient: A case report. AIDS Res Ther 2015; 12: 1-4.
Magan-Fernandez A, O'Valle F, Pozo E, Liebana J, Mesa F. Two cases of an atypical presentation of necrotizing stomatitis. J Periodontal Implant Sci 2015; 45: 252-256.
De Onis M, Monteiro C, Akre J, Clugston G. The worldwide magnitude of protein-energy malnutrition: An overview from the WHO global database on child growth. Bull World Health Organ 1993; 71: 703-712.
Enwonwu C O, Falkler W A, Idigbe E O et al. Pathogenesis of Cancrum Oris (Noma): Confounding interactions of malnutrition with infection. Am J Trop Med Hyg 1999; 60: 223-232.
McClain C J, Barve S S, Barve A, Marsano L. Alcoholic Liver Disease and Malnutrition. Alcohol Clin Exp Res 2011; 35: 815-820.
Phillips R S, Enwonwu C O, Falkler W A. Pro-versus anti-inflammatory cytokine profile in African children with acute oro-facial noma (cancrum oris, noma). Eur Cytokine Netw 2005; 16: 70-77.
Enwonwu C O, Falkler W A, Phillips R S. Noma (cancrum oris). Lancet 2006; 368: 147-156.
British Society of Periodontology. The Good Practitioner's Guide to Periodontology. Liverpool: BSP, 2016.
Ruggiero S L, Dodson T B, Fantasia J et al. Medication-Related Osteonecrosis of the Jaw - 2014 Update. J Oral Maxillofac Surg 2014; 72: 1938-1956.
Blot W J. Alcohol and Cancer. Cancer Res 1992; 52(7 Suppl): 2119s-2123s.
Public Health England. Delivering better oral health: an evidence-based toolkit for prevention. Third edition. London: Public Health England, 2017.
Scully C, Diz Dios P, Navdeep K. Special Care in Dentistry. 1st ed. Philadelphia: Churchill Livingstone, 2007.
Public Health England. Identification and Brief Alcohol Advice (IBA) for Dental Teams. 2020. Available online at https://portal.e-lfh.org.uk/myElearning/Index?HierarchyId=0_41_41&programmeId=41 (accessed June 2020).
Acknowledgements
The authors would like to thank Jo Adlington, Specialist in Special Care Dentistry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We, the authors, confirm we are happy to submit this article for consideration and have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Tkacz, K., Gill, J. & McLernon, M. Necrotising periodontal diseases and alcohol misuse - a cause of osteonecrosis?. Br Dent J 231, 225–231 (2021). https://doi.org/10.1038/s41415-021-3272-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41415-021-3272-9