Abstract
Objective To compare the clinical effectiveness of different frequencies of dental recall over a four-year period.
Design A multi-centre, parallel-group, randomised controlled trial with blinded clinical outcome assessment. Participants were randomised to receive a dental check-up at six-monthly, 24-monthly or risk-based recall intervals. A two-strata trial design was used, with participants randomised within the 24-month stratum if the recruiting dentist considered them clinically suitable. Participants ineligible for 24-month recall were randomised to a risk-based or six-month recall interval.
Setting UK primary dental care.
Participants Practices providing NHS care and adults who had received regular dental check-ups.
Main outcome measures The percentage of sites with gingival bleeding on probing, oral health-related quality of life (OHRQoL), cost-effectiveness.
Results In total, 2,372 participants were recruited from 51 dental practices. Of those, 648 were eligible for the 24-month recall stratum and 1,724 participants were ineligible. There was no evidence of a significant difference in the mean percentage of sites with gingival bleeding on probing between intervention arms in any comparison. For those eligible for 24-month recall stratum: the 24-month versus six-month group had an adjusted mean difference of -0.91%, 95% CI (-5.02%, 3.20%); the 24-month group versus risk-based group had an adjusted mean difference of 0.07%, 95% CI (-3.99%, 4.12%). For the overall sample, the risk-based versus six-month adjusted mean difference was 0.78%, 95% CI (-1.17%, 2.72%). There was no evidence of a difference in OHRQoL (0-56 scale, higher score for poorer OHRQoL) between intervention arms in any comparison. For the overall sample, the risk-based versus six-month effect size was -0.35, 95% CI (-1.02, 0.32). There was no evidence of a clinically meaningful difference between the groups in any comparison in either eligibility stratum for any of the secondary clinical or patient-reported outcomes.
Conclusion Over a four-year period, we found no evidence of a difference in oral health for participants allocated to a six-month or a risk-based recall interval, nor between a 24-month, six-month or risk-based recall interval for participants eligible for a 24-month recall. However, patients greatly value and are willing to pay for frequent dental check-ups.
Key points
-
This is the first national, multi-centre, pragmatic RCT in a primary care setting to evaluate the clinical, patient-centred and cost benefit of different recall intervals.
-
Traditional practice of scheduling six-monthly recall appointments for patients, regardless of their risk of developing dental disease, does not improve oral health. A variable risk-based recall interval is appropriate, is not detrimental to oral health, and is acceptable to patients and dentists.
-
Considering the impact of the COVID-19 pandemic limiting access to dental care, this study provides reassurance that, for appropriate patients, intervals between check-ups can be extended, based on an individual's risk, without detriment to oral health.
Similar content being viewed by others
Background
The original INTERVAL Trial report is published in Health Technology Assessment,1 and this paper summarises the clinical effectiveness results. Traditionally, patients have been encouraged to attend dental recall appointments at regular intervals of six months between appointments, irrespective of the individual's risk of developing dental disease. The principal function of the dental recall has been seen as the prevention and early detection of oral disease, in particular dental caries and periodontal disease.2 The recommendation of a six-month recall interval has become established practice in primary dental care in many countries,3,4,5,6,7 with dental check-ups at six-month intervals considered customary in the General Dental Service (GDS) in the United Kingdom since the inception of the National Health Service (NHS).
There has been a longstanding international debate regarding the clinical effectiveness and cost-effectiveness of recall intervals for routine dental check-up examinations.2,3,5 In 2004, the National Institute for Health and Care Excellence (NICE) published the guideline Dental recall: recall interval between routine dental examinations,8 designed to aid dentists in assigning individualised recall intervals to patients based on their risk of developing oral disease. The guideline recommends an adjustable recall interval for adults, ranging from a minimum of three months to a maximum interval of 24 months between recall appointments for patients who have repeatedly demonstrated an ability to maintain oral health. The recommendations are, however, based on low-quality evidence. Systematic reviews investigating this key question have reported limited evidence of poor overall quality, concluding that there is no evidence to support or refute the practice of encouraging patients to attend for dental check-ups at six-month intervals.9,10
The aim of the INTERVAL Trial was to compare the effectiveness of dental check-ups at different recall intervals for maintaining optimum oral health in dentate adults attending general dental practice.
Methods
Study design
The INTERVAL Dental Recalls Trial has been previously described in the published protocol.11 INTERVAL was a UK-wide multi-centre, parallel-group randomised controlled trial with blinded outcome assessment at four-year follow-up. Participants were randomised to attend for dental recall at one of three recall intervals - fixed-period six-month recall interval, adjustable risk-based recall (based on the NICE Guideline),8 and a fixed-period 24-month recall interval. Randomisation was conducted within two strata, with participants only randomised to the 24-month interval if considered clinically suitable by their recruiting dentist. Participants who were ineligible for 24-month recall were randomised to a risk-based or six-month recall interval.
Participants
Dentists in the UK who provide some NHS care and all dentate adults who had attended for a dental check-up at least once in the previous two years and received at least some of their dental treatment as an NHS patient were eligible for recruitment. Patients with uncontrolled medical conditions or at increased risk of bleeding were excluded.
Participating dentists represented a cross-section of practitioners operating across the UK in terms of urban or rural location, community-level socio-demographics, and fluoridated or non-fluoridated communities.
Randomisation and blinding
Eligible and consenting patient participants were clinically examined by their dentist to determine suitability for randomisation to the 24-month recall arm. This decision was based on routine clinical examination and risk assessment. Those considered eligible for the 24-month recall arm were randomised to one of the three intervention arms. Participants who were considered ineligible for the 24-month recall were randomised to either a risk-based or six-month recall interval.
Random allocation of participants to an intervention arm occurred via telephone, utilising the automated computer-generated randomisation system at the Centre for Healthcare Randomised Trials (CHaRT), University of Aberdeen, UK. Participants were randomised in equal proportions within each stratum according to a minimisation algorithm including: dentist, participant age, number of restored teeth, absence of gingival bleeding on probing, and participant exemption from dental charges. Due to the nature of the interventions, it was not possible to blind participants and dentists to allocated recall intervals.
Study interventions
Participants allocated to the fixed-period 24-month recall interval and the fixed six-month recall interval groups were invited to attend their dentist at the scheduled time intervals for a routine dental check-up. The content of this check-up remained as per current practice.
Participants allocated to the risk-based recall interval group attended their dentist at time intervals determined by the evidence-based process outlined in the 2004 NICE guideline on dental recall.8 The frequency of recall interval appropriate for an individual patient depends on the likelihood that specific diseases or conditions may develop or progress beyond the control of secondary prevention. It is therefore a multifaceted clinical decision that involves judgement and consideration of an individual's multiple risk and protective factors. The recommendation was that the recall interval range for adults should vary from three to 24 months, according to risk. The essential steps of the procedure and the risk factors collected at recall examinations are outlined in the NICE Guideline8 and summarised in Figure 1.
The NICE risk-based dental recall procedure and risk factors. © NICE 2004 Dental Recall - Recall interval between routine dental examinations. Available from https://www.nice.org.uk/guidance/cg19/evidence/full-guideline-pdf-193348909. All rights reserved. Subject to Notice of rights. Figure 1 is reproduced by kind permission from the National Institute for Health and Care Excellence (NICE). NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication
Training of the recruited dentists in determining risk-based recall interval according to the NICE Guideline, including setting and review of individualised patient recall intervals, was provided in the form of an online training package, developed specifically for this study. Dentists were instructed to complete this training before screening any potential patient participants for the trial, and annually throughout the trial follow-up period, with CPD credits provided for completing training.
Outcome measures
Clinical outcomes were recorded by blinded outcome assessors (n = 4) at the four-year follow-up period. Training was provided before clinical outcome collection and repeated halfway through trial outcome collection to ensure consistency of the assessment process. The primary clinical outcome was bleeding on probing at the gingival margin measured by running a University of North Carolina probe circumferentially around each tooth just within the gingival sulcus or pocket.12 After 30 seconds, bleeding was recorded as being present or absent on the buccal and lingual surfaces of each tooth and reported as the percentage of sites with bleeding. Dentists were advised that any periodontal screening or treatment, including scale and polish, should be delayed until after the trial outcome assessment.
Patient-reported outcomes were collected at baseline and annually by self-administered postal questionnaires. Oral health-related quality of life (OHRQoL) was the primary patient-reported outcome collected annually by postal questionnaires and measured using the Oral Health Impact Profile-14 (OHIP-14).13 The OHIP-14 is a 14-question oral health specific patient-centred measure referring to symptoms in the past 12 months which produces a score ranging from zero to 56, with worsening OHRQoL associated with higher scores.
Secondary outcomes were dental caries at the enamel and dentine thresholds, periodontal probing depth, calculus, dental anxiety,14 oral health-related knowledge and behaviours (including questions on toothbrushing duration and frequency, and interdental cleaning), oral health attitudes, generic quality of life, and satisfaction with dental care. Dentists' attitudes towards dental recall strategies were collected at baseline and at four years. Details on the assessment of secondary outcomes are provided in the INTERVAL Trial report.1
Trial oversight
The trial was overseen by a Trial Steering Committee (TSC) and an independent Data Monitoring Committee. A project management group took responsibility for the accuracy and completeness of the data, analyses, and reporting and for the fidelity of the study to the protocol.
Patient and public involvement
Prior to the start of the INTERVAL trial, patients provided input into the trial design and in recruitment and communication strategies. Patient contribution was also obtained in the design of the trial invitation and newsletters and in the layout of patient participant questionnaires. Members of the public were also involved in trial oversight through membership of the TSC, including contribution to interpretation of the trial findings and preparation of the final report.
Statistical analysis
An intention-to-treat analysis was performed for the pre-specified comparisons of six-month, risk-based and 24-month recall (for the group eligible for a 24-month recall) and risk-based versus six-month recall (for the group ineligible for a 24-month recall). Outcomes collected at year four were analysed using a generalised linear model with a random effect for dental practice; outcomes collected across the four years were analysed using a mixed effects model with two random effects: participant and practice. A time-by-treatment interaction term was included in the models. The appropriate effect sizes and 95% confidence intervals were derived. All analyses were adjusted for the minimisation variables, therefore alpha was set to 0.05, two-sided.
Missing items in scales were dealt with as recommended in the literature by their authors when recommendations were available. Otherwise, a complete case approach was used where, in the presence of any missing items in a patient's score, the score was considered missing. Continuous missing data at baseline were imput for modelling purposes.15 As a sensitivity analysis, and assuming a missing at random mechanism, multiple imputation was used to impute primary outcome data for patients with missing data.16
Subgroup analyses explored the possible modification of treatment effect by including a treatment-by-factor interaction in primary outcome models. Factors were: age (<45 years, 45-64, ≥65 years) and social class (exempt from payment; not exempt from payment). A post-hoc subgroup analysis by country was also included. Confidence intervals were calculated at 99%, therefore alpha was set to 0.01, two-sided.
Routine treatment data were obtained from the NHS Businesses Service Authority in England, Information Services Division in Scotland and Health and Social Care Northern Ireland Business Services Organisation in Northern Ireland for the time period of 2010 to 2018. They provided the number of treatment claims for dental check-ups made by dentists for each participant. This data was collected from the dental practice records for participants recruited in Wales.
Sample size
A study with 750 participants in each arm could detect a difference in bleeding scores of 4.5% at 90% power and 5% significance level, and likewise detect a difference of 0.17 of the standard deviation of the OHIP-14.17 For the caries clinical outcome, assuming a standard deviation of 3.5, a study with 750 participants per arm could detect a 20% relative shift in white spot lesions from 3.3 to 3.9 at 90% power and 5% significance.18 Our sample size calculations indicated we need to randomise 705 participants to stratum 1 (235 in each arm) and 1,030 to stratum 2 (515 in each arm). Assuming an intra-cluster correlation of 0.03, the trial had 80% power to detect a difference of 4.5% of gingival sites bleeding on probing. In the power calculation we have assumed a loss to follow-up for dentists of 10% based on the observed rates of 12% and 9% in two recent large, multi-centre practice-based RCTs.19
Economic evaluation
A within-trial economic evaluation was conducted over the four-year trial time horizon. Economic evaluations typically take the form of cost-utility (that is, cost per quality adjusted life year [QALY]). However, in the context of dentistry, there are concerns that generic EQ-5D-based QALYs lack the sensitivity to capture the processes and outcomes of care that are of value to patients and decision makers. Different perspectives of benefits were therefore evaluated (willingness to pay [WTP]) for dental recall interval and dental health outcomes, WTP for dental health outcomes only, and QALYs. The perspectives for costs were NHS dental costs, all NHS costs and participant costs. Costs were collected using dental claims data and annual participant completed questionnaires. QALYs were calculated using responses to the annual participant completed EQ-5D-3L questionnaire and valued according to UK general population tariffs. An online discrete choice experiment (DCE) with a nationally representative sample of the UK general population was used to estimate WTP tariffs which were then mapped to frequency of recall interval, bleeding on brushing and caries experience observed in the trial. Multiple imputation was used to address missing data and incremental costs and benefits were estimated using generalised linear regression models.
Results
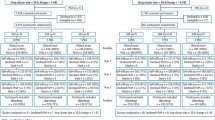
Recruitment took place between July 2010 and July 2014 and follow-up closed in April 2018. The flow of participants in the trial is shown in Figures 2 and 3. Across 51 dental practices, 2,372 participants were recruited. To the 24-month recall stratum, 648 were recruited; 217 allocated to the risk-based recall, 216 to the 24-month recall and 215 to the six-month recall. In the ineligible for 24-month recall stratum, 1,724 participants were recruited; 861 allocated to risk-based recall and 863 to the six-month recall. In total, 1,078 participants received a risk-based recall and 1,078 received a six-month recall. Participant characteristics at baseline are shown in Table 1. The average age of participants was 45 years, the majority were women (57%) and regular dental attenders. The majority of the study population reported brushing their teeth at least twice per day (81%) and 38% used an electric toothbrush. Mean OHIP-14 scores (0-56 scale) were low indicating good OHRQoL in both strata - 4.4 in the eligible for 24-month recall stratum and 5.7 in the ineligible stratum. Overall, participants in the ineligible for 24-month recall stratum were older, self-reported to attend the dentist more regularly and had higher OHIP scores than those in the eligible stratum. There were no important differences or imbalances across randomised groups in each of the eligibility strata.
Primary outcomes
Overall, 416 (64%) participants within the eligible for 24-month recall stratum attended a follow-up appointment and 460 (71%) completed a year four questionnaire. Within the ineligible stratum, 1,208 (70%) participants attended a follow-up appointment and 1,305 (76%) completed a year four questionnaire.
Participants in the eligible for 24-month recall stratum were assigned recall appointments at a mean of 13 months (risk-based), 24 months (24-month recall) and seven months (six-month recall). Participants in the ineligible for 24-month recall stratum were assigned recall appointments at a mean of nine months (risk-based) and 8.5 months (six-month recall). According to routinely collected data, over the four-year follow-up period, participants eligible for the 24-month recall stratum who had a clinical outcome assessment had a mean of 3.7 (SD 1.9) check-ups in the risk-based arm, 2.5 (SD 2.2) in the 24-month and 5.1 (SD 3.7) in the six-month recall arm. Participants who were ineligible for the 24-month recall who had a clinical outcome assessment had a mean of 5.0 (SD 2.3) check-ups during the trial in the risk-based arm and 5.4 (SD 2.0) in the six-month arm.
Within the eligible for 24-month recall stratum, the percentage of sites bleeding at four years were: six-month 35.6% (SD 21.7), risk-based 35.6% (SD 19.1), and 24-month 34.4% (SD 20.1). Within the ineligible for 24-month recall stratum, the percentage of sites bleeding at four years were: six-month 32.8% (SD 22.1) and risk-based 33.4% (SD 22.2).
The treatment effects for the primary outcomes and secondary clinical outcomes are presented in Table 2. At four years, there was no evidence of a significant difference in gingival bleeding on probing between the groups in any comparison: the 24-month group versus six-month had an adjusted mean difference of -0.91%, 95% CI (-5.02%, 3.20%), p-value = 0.66; the risk-based versus six-month recall had an adjusted difference of -0.98% 95% CI (-5.05%, 3.09%), p-value = 0.64; the 24-month versus risk-based had an adjusted mean difference of 0.07%, 95% CI (-3.99%, 4.12%), p-value = 0.97. For the overall sample, the risk-based recall versus six-month recall had an adjusted mean difference of 0.78%, 95% CI (-1.17%, 2.72%), p-value = 0.43. Multiple imputation was used for the primary clinical outcome (gingival bleeding on probing) for sensitivity analysis, which did not change the interpretation of the results.
Within the eligible for 24-month recall stratum, the mean OHIP-14 scores were: six-month 4.8 (SD 6.2), risk-based 4.1 (SD 5.7), and 24-month 4.8 (SD 6.4). Within the ineligible for 24-month recall stratum, the mean OHIP-14 scores were: six-month 5.8 (SD 8.3) and risk-based 5.5 (SD 6.8). Table 3 summarises results for the trial primary outcomes and secondary clinical outcomes. The results for other secondary outcomes are presented in the online Supplementary Table 1.
There was no evidence of a difference across comparisons for OHRQoL between the groups in any comparison: the 24-month group versus six-month had an effect size of -0.24-95% CI (-1.55, 1.07), p-value = 0.72; the risk-based versus six-month recall had an effect size of -0.61-95% CI (-1.93, 0.71), p-value = 0.37; the 24-month versus risk-based had an effect size of 0.37-95% CI (-0.95, 1.69), p-value = 0.58. For the overall sample the risk-based recall versus six-month recall had an effect size of -0.35, 95% CI (-1.02, 0.32), p-value = 0.30.
Secondary outcomes
The remaining treatment effects for the secondary clinical outcomes are presented in the same Table 3 and secondary patient-reported outcomes in the online Supplementary Table 2. There was no evidence of a clinically meaningful difference between the groups in any comparison in either eligibility stratum for any outcome.
Subgroup analyses
Figures 4 and 5 show the means and 99% confidence intervals for the differences in gingival bleeding on probing at four years in the subgroups for recall frequency and stratum respectively. In England, in the eligible to a 24-month recall stratum, participants randomised to a six-month recall showed a significant improvement compared with those randomised to a risk-based recall (mean difference 4.98-95% confidence interval (1.14, 8.83), p-value <0.001). There was no evidence of treatment modification among the pre-specified subgroups (Figures 4 and 5).
Dentists' attitude regarding a 24-month recall improved between baseline and follow-up, as did their attitude regarding six-month recall. Where dentists considered at least one patient eligible for the 24-month recall interval (n = 40), a slight increase was seen in their perceived ability to judge risk. Where dentists did not consider any patient eligible for a 24-month recall (n = 6) a decrease in their perceived ability to judge risk was seen.
Economic evaluation
The economic evaluation results varied depending on the perspective of benefits considered.
The DCE showed that the general population were willing to pay to avoid progressive levels of dental decay and bleeding gums but were also willing to pay for (and highly valued) more frequent recalls. Including all sources of utility to the general population (health and non-health), six-monthly recalls generated the greatest net benefit (cost less WTP) and the finding was consistent across sensitivity analyses undertaken.
When restricting the scope of benefit valuation to dental outcomes only (that is, WTP for bleeding on brushing and caries experience only), 24-month recall is the most likely optimal strategy, with a probability of positive net dental health benefit (cost less WTP for dental health outcomes) ranging between 65% and 99% across a range of sensitivity analyses conducted. Results are driven by potential for significant cost savings when considering the cost burden to participants and the NHS combined, with no evidence of a difference in clinical outcomes. For the trial population as a whole (including both eligible and ineligible for 24-month recall stratum), there is substantial uncertainty regarding the most efficient strategy (risk based or six-monthly) to maximise dental health benefit. Risk-based recalls were more likely to generate positive net dental health benefit when a wider perspective (NHS + participant) of the costing analysis was considered.
The optimal recall was unclear when evaluating cost per QALY gained, due in part to the lack of sensitivity of the generic EQ-5D to capture dental outcomes. In the combined analysis across both trial strata, no strategy achieved a probability of cost-effectiveness greater than 70% at a threshold value of society's WTP for a QALY gain of £20,000. The probability of cost-effectiveness was higher for the 24-month recall strategy in the analysis restricted to the eligible stratum due to the potential for cost savings associated with longer recall intervals for the minority of participants deemed eligible for them.
Discussion
This is the first national, multi-centre, pragmatic RCT in a primary care setting to evaluate the clinical, patient-centred and cost benefit of different recall intervals. The INTERVAL trial investigated the implementation of a risk variable approach to recall as recommended in the NICE guideline on dental recall.8 The guideline considers the effect of dental recalls on patients' wellbeing, general health and preventive habits, as well as caries incidence, the need for restorative treatment, patients' periodontal health, maintenance of dentition, and avoidance of pain and dental anxiety. It aims to improve or maintain patients' quality of life and reduce morbidity associated with oral disease. This guideline was initially published in 2004 and most recently reviewed in 2018, confirming there was no emerging evidence to change the recommendations. Challenges to assumed routine dental practice such as the six-month dental recall and the benefit of regular scale and polish were voiced as early as 1977.2 The mantra of a six-month recall has been in existence for decades and trying to establish the scientific basis for a six-month or a variable risk-based recall interval was the reason for this trial. Contemporary healthcare supports a patient-centred, appropriate, preventive, and compassionate approach and a dental recall visit is the opportunity for oral disease to be diagnosed early and preventive advice and therapy to be provided. The aim of this RCT in primary care dental practice was to provide evidence for the benefit or harm of dental check-ups at different recall intervals on maintaining oral health.
This study has shown that a variable risk-based recall interval is appropriate, is not detrimental to oral health and is acceptable to patients and dentists. Over a four-year period, we found no difference in oral health for patient participants allocated to a six-month or a variable risk-based interval. Nor did we find a difference between the intervals of 24-month, six-month and risk-based for the 30% of adults considered suitable to be recalled at 24 months by their dentist. Extending the recall from six-months had no effect on patient-reported OHRQoL and the participants were satisfied with being allocated to a recall interval based on risk. No evidence of a difference was found in any of the secondary clinical outcomes measured between the three recall intervals for those eligible for 24-month recall or the overall six-month and risk-based groups. The secondary clinical outcomes were coronal caries measured at three thresholds (initial, moderate and extensive), presence of root surface caries, mean periodontal probing depth and presence of calculus.
Participants deemed eligible to be allocated to a 24-month recall had, on average, a better OHRQoL score than those deemed ineligible. Participants deemed ineligible were more likely to identify themselves as regular attenders. This suggests participating dentists were already reliably assessing patients' oral health risk and our study shows potential for cost savings to the NHS and participants by extending dental recalls to 24 months for the minority of patients who are deemed eligible. The Cochrane review on recall intervals has now been updated to include results from the INTERVAL trial.9 This timely update has led to a change in the conclusions and increase in the level of certainty of the evidence.
A recent correspondence article in the Lancet commented on the extraordinary impact of the COVID-19 global pandemic on dental services worldwide, which essentially closed down for five months, limiting access even to emergency dental care.20 As dental services tentatively re-open, with guidance on safety procedures to follow, the issue of reduced access to dental care may be a challenge for dental services and patients for some time. There is therefore an impetus to reform dental services to meet the challenges of prioritising care for high-need demographics and pursuing a minimally invasive prevention-orientated practice in light of the restrictions on aerosol generating procedures. Serious consideration must also be given to ceasing ineffective treatments that utilise valuable resources without improving health outcomes. The results of this study provide important supporting evidence that intervals between dental recall appointments can be extended beyond six months without detriment to the oral health of patients.
Limitations
The decision to allocate a participant to the risk-based recall arm was made by the recruiting dentist. Training on determining recall interval based on an individual's risk of developing dental disease was provided according to NICE guidelines, however, the process of assessing risk and the factors considered in making this decision was not operationalised for collection. Similarly, factors considered by dentists in determining eligibility to the 24-month recall arm were not assessed and collected.
INTERVAL had a dropout of 25-30% in the questionnaire data and 30-37% in attendance of follow-up appointments, a higher value than expected at the design stage of the trial. However, the dropout rates were balanced between the arms, and sensitivity analyses using multiple imputation showed the results were robust.
Conclusions
This trial compares the clinical effectiveness of frequency of dental recall appointments in primary dental care over four years. It comes to the controversial conclusion that there is no clinical benefit of a six-monthly recall compared to a risk-based recall or 24-month recall in those patients considered eligible. The absence of evidence of a difference between the three recall strategies therefore indicates a variable risked-based recall interval can be supported as it is not detrimental to oral health. The current evidence therefore suggests that current practice of scheduling six-monthly recall appointments for patients, regardless of their risk of developing dental disease, does not improve oral health. This could be considered an inefficient use of scarce NHS resources, adding unnecessary patient costs for no gain in dental health outcomes, particularly for the subgroup of patients who are deemed suitable for longer 24-month recall intervals. However, six-monthly recalls are highly valued by the general population, and moving towards a personalised, variable recall strategy will require the cooperation of healthcare policymakers, clinicians' practices and patients.
References
Clarkson J E, Pitts N B, Goulao B et al. Risk-based, 6-monthly and 24-monthly dental check-ups for adults: the INTERVAL three-arm RCT. Health Technol Assess 2020; 24: 60.
Sheiham A. Is there a scientific basis for six-monthly dental examinations? Lancet 1977; 310: 442-444.
Clarkson J E, Amaechi B T, Ngo H, Bonetti D. Recall, reassessment and monitoring. Monogr Oral Sci 2009; 21: 188-198.
Frame P S, Sawai R, Bowen W H, Meyerowitz C. Preventive dentistry: Practitioners' recommendations for low-risk patients compared with scientific evidence and practice guidelines. Am J Prev Med 2000; 18: 159-162.
Kay E J. How often should we go to the dentist? BMJ 1999; 319: 204-205.
Patel S, Bay R C, Glick M. A systematic review of dental recall intervals and incidence of dental caries. J Am Dent Assoc 2010; 141: 527-539.
Gussy M, Bracksley S, Boxall A. How often should you have dental visits? Aust Healthc Hosp Inst 2013; 10: 1-6.
National Institute for Health and Care Excellence (NICE). Dental Recall: Recall Interval between Routine Dental Examinations. NICE Clin Guide 19 2004; DOI: 10.1039/j19690001101.
Riley P, Worthington H V, Clarkson J E, Beirne P V. Recall intervals for oral health in primary care patients. Cochrane Database Syst Rev 2013: DOI: 10.1002/14651858.CD004346.
Davenport K, Salas C L, Fry-Smith A, Bryan S, Taylor R, C Elley C TW. The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation. Health Technol Assess Database 2010; 7: 1-127.
Clarkson J E, Pitts N B, Bonetti D et al. INTERVAL (investigation of NICE technologies for enabling risk-variable-adjusted-length) dental recalls trial: a multicentre randomised controlled trial investigating the best dental recall interval for optimum, cost-effective maintenance of oral health in dentate adults attending dental primary care. BMC Oral Health 2018; 18: 135.
Löe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol 1967; DOI: 10.1902/jop.1967.38.6.610.
Slade G D. Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol 1997; 25: 284-290.
Humphris G M, Freeman R, Campbell J, Tuutti H, D'Souza V. Further evidence for the reliability and validity of the Modified Dental Anxiety Scale. Int Dent J 2000; 50: 367-370.
White I R, Thompson S G. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005; 24: 993-1007.
White I R. Strategies for handling missing data in randomised trials. Trials 2011; DOI: 10.1186/1745-6215-12-S1-A59.
Clarkson J E. The effectiveness of enhanced oral health advice and instruction upon patient oral hygiene, knowledge, and self-reported behaviour. Chief Sci Off 2005.
Chesters R K, Pitts N B, Matuliene G et al. An abbreviated caries clinical trial design validated over 24 months. J Dent Res 2002; 81: 637-640.
Clarkson J E, Turner S, Grimshaw J M et al. Changing Clinicians' Behaviour: a Randomized Controlled Trial of Fees and Education. J Dent Res 2008; 87: 640-644.
Watt R G. COVID19 is an opportunity for reform in dentistry. Lancet 2020; 396: 462.
Acknowledgements
We would like to acknowledge the participating dental practice teams and patients without whose valuable contribution this study could not have taken place. The Health Services Research Unit and the Health Economics Research Unit are funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorates. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. Ethics approval for the trial was given by the Fife and Forth Valley Research Ethics Committee on 13 January 2009 and 20 September 2011 (Research Ethics Committee reference 09/SO501/1). The trial was registered with the ISRCTN (reference number ISRCTN95933794).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
None to disclose. This trial was funded by the NIHR HTA programme [project numbers 06/35/05 (Phase I) and 06/35/99 (Phase II)].
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Clarkson, J., Pitts, N., Fee, P. et al. Examining the effectiveness of different dental recall strategies on maintenance of optimum oral health: the INTERVAL dental recalls randomised controlled trial. Br Dent J 230, 236–243 (2021). https://doi.org/10.1038/s41415-021-2612-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41415-021-2612-0
This article is cited by
-
Could retaining the dental recall interval save NHS dentistry?
British Dental Journal (2024)
-
A two-arm, randomised feasibility trial using link workers to improve dental visiting in people with severe mental illness: a protocol paper
Pilot and Feasibility Studies (2023)
-
Long-term caries prevention of dental sealants and fluoride varnish in children with autism spectrum disorders: a retrospective cohort study
Scientific Reports (2022)
-
A qualitative exploration of decisions about dental recall intervals - Part 1: attitudes of NHS general dental practitioners to NICE guideline CG19 on the interval between oral health reviews
British Dental Journal (2022)
-
Oral health-related quality of life outcomes in a randomized clinical trial to assess a community-based oral hygiene intervention among adults living in low-income senior housing
Health and Quality of Life Outcomes (2021)