Abstract
Periodontitis and gingivitis remain two of the most common diseases that affect the oral cavity. As they are caused by plaque, effective oral hygiene, elimination of plaque-retentive factors and successful periodontal treatment will result in resolution of gingival and periodontal inflammation. Certain systemic diseases can have a clinical appearance similar to periodontal diseases or exacerbate existing periodontitis/gingivitis and vice versa. This paper aims to provide the dental practitioner with an understanding of the manifestations of systemic diseases to the periodontium and highlights elements in the clinical assessment, which will aid in establishing a correct diagnosis. Additional anamnestic and clinical clues are important for distinguishing between plaque-induced and non-plaque-induced lesions. The first part of this compendium covers immune-mediated and hereditary conditions as causes of gingival lesions, which can resemble those caused by dental plaque. The different conditions are presented concisely and exemplified by clinical photographs. Dental practitioners should be aware of the various manifestations of systemic diseases to the periodontium in order to offer appropriate diagnosis and treatment, which can reduce both patient morbidity and mortality.
Similar content being viewed by others
Key points
-
Provides dental practitioners with an understanding of the manifestation of systemic diseases to the periodontium.
-
Highlights elements in the clinical assessment which will aid in establishing a diagnosis.
-
Presents different conditions concisely and outlines these using clinical photographs.
Introduction
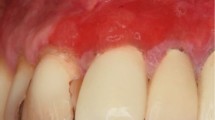
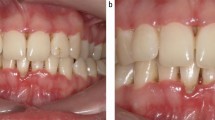
Periodontitis is a complex inflammatory disease that affects the soft and hard periodontal structures (Fig. 1a). It is a plaque-related disease initiated by bacteria, and driven by the host response, genetic predisposition and environmental risk factors. Periodontitis, if left untreated, often leads to progressive destruction of the tooth supporting tissues and tooth loss.1 Typical clinical findings are pocketing and/or gingival recession. Gingivitis on the other hand is characterised by redness and swelling of the keratinised gingiva adjacent to the teeth (Fig. 1b). In plaque-induced gingivitis, these symptoms resolve after plaque control. Unlike periodontitis, gingivitis is therefore a reversible disease. There are, however, cases that do not respond to classic plaque control regimes, including establishment of appropriate oral hygiene, scale and polish, administration of chemical plaque control and elimination of plaque-retentive factors. In cases of non-resolving gingival inflammation the underlying cause is often of a systemic nature and careful examination of the oral cavity for other mucosal changes may reveal findings indicative of an underlying systemic condition.2
Prompt and accurate diagnosis of underlying systemic disease facilitates appropriate referral, investigation and management. It is also important to acknowledge that non-plaque-induced gingival lesions are often colonised and infected by oral bacteria whereby gingival and periodontal inflammation can additionally contribute to disease activity. Thus, plaque control measures may ameliorate such lesions but they do not resolve until the systemic cause is targeted. The National Health Service (NHS) UK recommends that patients are seen for dental check-ups between every 3 to 24 months, depending on their oral health and needs. Because of the regular frequency of dental check-ups dental practitioners are in a unique position to facilitate an early diagnosis of systemic diseases, which can manifest in the oral cavity.
This compendium focuses on non-plaque-induced and non-resolving lesions of the keratinised gingiva in dentate patients and aims to aid the practitioner to distinguish those systemic diseases that might be confused with plaque-induced gingival lesions. However, the authors have also included other systemic and related conditions, which commonly manifest to the gingivae, with the aim to provide the reader with a guide to establishing a differential diagnosis. The non-plaque-induced gingival/periodontal lesions described herein are also in accordance with the recently published Consensus Report of Workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions.3
The systemic underlying conditions along with oral clinical photographs, demographic data and clinical descriptions are presented in a summary table. In this article, part 1 of this compendium, we review immune mediated, autoinflammatory, and hereditary gingival lesions. In part 2 of the compendium we go on to review cancer related gingival lesions, infective and other causes of gingival inflammation.
1. Anatomy and terminology
1.1 Anatomy of the periodontium
The periodontium consists of five types of tissues:
-
1.
Free gingiva, the tissue collar around the tooth, which forms the gingival crevice and is lined with epithelium, which is non-keratinised on the tooth-facing side and keratinised on the oral cavity-facing side. It is attached to the tooth at its base by the epithelial attachment, the junctional epithelium. The interdental free gingiva is referred to as the interdental papilla, which is knife-edged under healthy conditions
-
2.
Attached gingiva, the tissue adjacent to the free gingiva, which is keratinised and firmly attached to the underlying bone structure
-
3.
Alveolar mucosa, the area beyond the mucogingival junction, which is less firmly attached and non-keratinised. The mucogingival junction, a scalloped line, divides attached gingiva and alveolar mucosa
-
4.
Alveolar bone consisting of dense cortical plates and cancellous bone as well as of the lamina dura, the wall of the socket
-
5.
Periodontal ligament, which is attached to the lamina dura as well as to the cementum of the tooth, consists of apical, oblique, horizontal, alveolar crest and interradicular fibres as well as of blood and lymphatic vessels and nerves.
1.2 Plaque-induced gingival lesions
The oral cavity is habitat to several hundred microbial species including bacteria, viruses and fungi. The periodontium is a unique point of challenge in the body, as the tooth-bone interface constitutes a potential port of entry for microorganisms, whereas all other surfaces of the gastro-intestinal tract provide a closed epithelial layer, which eliminates colonising bacteria by epithelial shedding. Thus, if microorganisms on tooth surfaces are allowed to overgrow in the form of a biofilm (plaque), or calculus, an inflammatory response of the periodontal and gingival tissues is initiated. Plaque-induced gingivitis is characterised by redness and swelling, but is fully reversible by eliminating biofilms from the tooth surfaces. Periodontitis is accompanied by pocket formation in the area of the junctional epithelium, in an attempt by the host immune cells to eliminate the biofilm by releasing toxic enzymes, proteins and reactive oxygen species. Nevertheless, the pocket environment provides excellent growth conditions for anaerobic bacteria, thereby driving inflammation and host-mediated tissue destruction. Only if biofilms and calculus are effectively removed from the pockets can tissue repair take place. An important distinguishing feature of plaque-induced gingival lesions is that, with the exception of necrotising and ulcerative forms, they are usually painless whereas non-plaque-induced gingival lesions are often symptomatic.
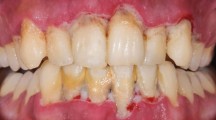
1.3 Gingival enlargement
Gingival enlargement or overgrowth (Fig. 2) is encountered in a variety of systemic and related conditions including orofacial granulomatosis (see section 2.3.1), granulomatosis with polyangiitis (see section 2.4), neutropenia (see section 2.6), plasma cell gingivitis (see section 2.7), hereditary gingival fibromatosis and idiopathic gingival enlargement (see section 3.1), drug-induced gingival enlargement (see Part 2 of this series, section 3.1), scurvy (see Part 2: section 3.2) and mouth breathing (see Part 2: section 4.1). It can also occur in plaque-induced gingivitis and periodontitis, especially in adolescents, during pregnancy, and in necrotising/ulcerative forms of these diseases. Distinguishing the cause can therefore be challenging but the medical history, extent of gingival swelling, presence of inflammation, and response to periodontal intervention can help in differentiating a diagnosis. An important distinguishing feature of some but not all of the systemic conditions in which gingival enlargement occurs is involvement of both the attached and unattached gingivae sometimes termed a 'full-width' or 'full-thickness' gingivitis. Involvement of the unattached gingivae can be seen in orofacial granulomatosis, plasma cell gingivitis, and polyangiitis with granulomatosis.
1.4 Desquamative gingivitis
Desquamative gingivitis (DG) (Fig. 3) is a clinical descriptor rather than a diagnosis. Several mucocutaneous pathologies, including pemphigus vulgaris (see section 2.1), pemphigoid and erythema multiforme (see section 2.2), lichen planus and lupus erythematosus (see section 2.5), allergic reactions (see section 2.7) and drug-induced lesions (see Part 2: section 3.1), can result in desquamative gingivitis. Widespread desquamation and/or erosion of the attached gingiva of the teeth is considered the chief characteristic feature of desquamative gingivitis, it often affects the buccal and labial gingivae but can also occur in the palatal and lingual gingivae. Similarly to full-thickness gingival enlargement, DG is often but not always contiguous with involvement of the adjacent unattached gingivae and sulcus mucosa, which should alert the practitioner to the possibility of an alternative diagnosis. In addition to the underlying immune-inflammatory condition driving the desquamative gingivitis dental plaque is an important aggravating factor. It is often difficult to distinguish the cause of DG on purely clinical grounds and histology and immunologic testing are usually necessary to establish a diagnosis.4
2. Immune-mediated and autoinflammatory gingival lesions
2.1 Pemphigus vulgaris
Pemphigus vulgaris (PV) is an autoimmune blistering disease affecting the skin and mucous membranes (Fig. 4, Table 1). Oral lesions usually appear before skin lesions, and in many cases the oral cavity remains the only site affected. Autoantibodies are directed against desmosomes (epithelial adhesion proteins) that bind stratified squamous epithelial cells together.5 In common with other vesiculobullous diseases oral PV presents as shallow oral ulceration, preceded by fluid-filled blisters, which are rarely seen clinically due to their fragility. PV can affect any mucosal surfaces and desquamative gingivitis is a common feature. The preliminary diagnosis is based on the clinical presentation and confirmed by histology. PV can be life threatening, particularly in cases with extensive cutaneous involvement, by means of secondary infection leading to septicaemia. Early diagnosis and management can prevent this complication.6 However, treatment particularly with high dose corticosteroids can lead to significant and potentially life-threatening complications including diabetes, cardiovascular disease, peptic ulcers, adrenal insufficiency and infection.7
2.2 Pemphigoid
Pemphigoid is a group of vesiculobullous diseases and is a type of immune-mediated sub-epithelial blistering disease (IMSEBD). These diseases are mediated by a variety of autoantibodies that target the extracellular components that link the basement membrane to basal epithelial cells or to the lamina propria.8 A number of IMSEBD can affect the oral cavity including dermatitis herpetiformis, toxic epidermal necrolysis/erythema multiforme, chronic bullous dermatosis of childhood, and pemphigoid variants. Pemphigoid variants include gestational pemphigoid, bullous pemphigoid, and mucous membrane pemphigoid (MMP). Additionally, acquired epidermolysis bullosa and linear IgA disease are now thought to be variants of MMP.9,10 Of the pemphigoid variants, mucous membrane pemphigoid (MMP) most commonly affects the oral cavity, with approximately 85 percent of affected patients having oral involvement (Fig. 5, Table 1). When affecting the gingiva, mucous membrane pemphigoid commonly presents as desquamative gingivitis.11 Additionally MMP can affect a variety of extraoral sites including the ocular, nasal, nasopharyngeal, anogenital, laryngeal and oesophageal mucosa, and can also rarely involve the skin. Ocular involvement is of particular concern as this results in scarring which can eventually lead to blindness in the affected eye.12 Although this disorder has an unclear aetiology, pemphigoid-like lesions can occasionally be drug-induced. There is no standard treatment protocol for the management of patients with MMP. The treatment strategies depend on the age of the patient, comorbidities, severity of the disease and the site involved, and may include the administration of corticosteroids, antimetabolites or antibiotics. Plaque control measures can play an important role in controlling oral inflammation in this condition.13
2.3 Orofacial granulomatosis and related conditions
Orofacial granulomatous inflammation can occur in a variety of disparate conditions including inflammatory bowel disease, particularly Crohn's disease, sarcoidosis, Melkersson-Rosenthal syndrome, tuberculosis and other mycobacterial infections. Cases can also occur without any apparent association with an underlying systemic disease in which case it might be thought of as idiopathic orofacial granulomatosis.14 Although the aetiopathogenesis of these conditions is varied or indeed unknown, we have grouped these disorders together based on their common clinical presentation to facilitate discussion.
2.3.1 (Idiopathic) orofacial granulomatosis
Orofacial granulomatosis (OFG) is a rare, granulomatous inflammatory disorder that is characterised by persistent enlargement of the soft oral and perioral tissues and has an unknown aetiology. Importantly, an onset of OFG during childhood may predict future development of Crohn's disease.15 Affected patients typically present with persistent painless swelling of the lips (cheilitis granulomatosa), indurated tag-like lesions, cobblestoning, linear ulceration and mucogingivitis. One of the characteristic features of the hyperplastic gingivitis seen in OFG is that it affects the attached and unattached gingivae (full-thickness gingivitis). The mechanism of the enlargement is granulomatous inflammation. The best treatment of this condition and its prognosis are uncertain.
Melkersson-Rosenthal syndrome, a neurological disorder, is characterised by orofacial swelling analogous to OFG, lower motor neurone facial palsy, and less commonly, fissured tongue. The facial palsy tends to be unilateral and may be indistinguishable from Bell's palsy.16
2.3.2 Inflammatory bowel diseases
Oral manifestations of the inflammatory bowel diseases Crohn's disease (CD) and ulcerative colitis (UC) have been reported to affect up to 60% of the affected patients.17,18 Crohn's disease is a polygenic autoinflammatory syndrome and which can affect any part of the gastrointestinal tract, including the mouth, although a majority of cases involve the terminal ileum. Orofacial granulomatosis is the commonest orofacial manifestation of this disease, but aphthous stomatitis is also occasionally seen in both CD and UC; however, this is a non-specific finding.
Pyostomatitis vegetans is most commonly encountered in patients with UC but has occasionally been reported in patients with CD. It is a chronic mucocutaneous ulcerative disorder consisting of multiple miliary white or yellow pustules with an erythematous and oedematous mucosal base (Fig. 6, Table 1). The pustules can rupture and coalesce to form linear or 'snail-track' ulcers. The most frequently involved regions of the oral cavity are the labial gingiva and the labial and buccal mucosa, the tongue and floor of the mouth are rarely involved but it can affect almost all parts of the oral cavity.18,19
2.3.3 Sarcoidosis
Sarcoidosis is a chronic systemic disease of unknown aetiology and is characterised by an accumulation of epithelioid granulomas. Sarcoidosis affects multiple organs, especially the lungs, lymph nodes, skin and eyes. The salivary glands are frequently involved, sometimes resulting in xerostomia or bilateral parotid swelling, the combination of parotid swelling, fever, and anterior uveitis is known as uveoparotid fever or Heerfordt syndrome. Oral sarcoidosis is rare and the prevalence is unknown, while the treatment remains controversial and may range from oral glucocorticoids to surgical excision.20 Oral mucosal presentations are varied but include orofacial granulomatosis and localised erythematous swelling which can affect any oral mucosal site including the gingivae (Fig. 7, Table 1).21
2.4 Granulomatosis with polyangiitis
Granulomatosis with polyangiitis, previously known as Wegener's granulomatosis, is a rare systemic disorder that can affect all areas of the body, including the oral cavity. It is a form of vasculitis that affects small- and medium-size vessels in many organs. Those with the disease require long-term immunosuppression, as otherwise this condition is lethal. The typical oral manifestations occur as gingival erosions and ulcerations, which often appear hyperplastic, leading to the typical clinical presentation of a 'strawberry gingivitis'. Oral involvement in granulomatosis with polyangiitis has been reported in 10-62% of cases, but oral involvement as the first sign of the disease is found in only 2% of cases (Fig. 8, Table 1).22
2.5 Oral lichen planus
Lichen planus is a mucocutaneous disorder, which commonly affects the skin and skin appendages (including scalp and nails), oral mucosa, and genitalia. Oesophageal, ocular, laryngeal, nasal, and urinary tract mucosa involvement have also rarely been reported. The epithelial damage is immunologically mediated by cytotoxic T-cells directed against basilar keratinocytes and results in vacuolar degeneration and lysis of basal cells. It is not a classical autoimmune disorder and is probably best typified as an inflammatory disease. Its aetiology is poorly understood but a variety of factors have been suggested including genetic, physiological, systemic disease, and hypersensitivity but there is currently no consensus.23 Oral lichen planus (OLP) is one of the most common oral mucosal diseases.24 Gingival involvement (desquamative gingivitis) is very frequently observed, and the disease is characterised by a high variability in clinical appearance and symptoms (Fig. 9, Table 1). There are several recognised clinical types including reticular, papular, plaque-like, atrophic, erosive/ulcerative and, rarely, bullous variants. Additionally, oral lichenoid lesions including lichenoid contact lesions, lichenoid drug reactions, lichenoid lesions of graft versus host disease and lichenoid interface mucositis, seen in certain forms of lupus, can confuse the diagnosis. In common with many immune-inflammatory oral mucosal diseases, a synergy with periodontal inflammation exists, which can influence disease activity. Therefore, patients with OLP require appropriate periodontal management, in combination with systemic evaluation, and topical or systemic immunosuppression/modulation.25
2.6 Neutropenia
The neutrophil is a polymorphonuclear immune defence cell and is the principal cell of the innate immune system. It therefore plays an important role in responding to infection through a diverse array of killing mechanisms including degranulation, respiratory burst resulting in the release of reactive oxygen species, and extrusion of their cytosol and nuclear chromatin as neutrophil extracellular traps. Additionally, as the first responder to microbial ingress the neutrophil helps orchestrate the immune-inflammatory response to infection.26
As stated in section 1.2, the oral cavity is a unique environment in the body as there is a break in the integument at the tooth-bone/mucosa-tooth interface, which constitutes a potential direct entry port for microorganisms. As such the oral cavity has long been recognised as a site of particular relevance in individuals with immune defects, and this is commonly the case in those with neutropenia. Neutropenia is defined as a paucity or defective function of neutrophils, and there are several causes of neutropenia including primary forms (chronic benign neutropenia, cyclical neutropenia, and other congenital and familial neutropenias) and secondary forms (iatrogenic, aplastic anaemia, leukaemia, connective tissue disease, malignancies and infections) all of which result in underproduction of neutrophils in the bone marrow. Neutropenia can also result from increased destruction of neutrophils (hypersplenism and immune neutropenia) and is rarely due to congenital immune defects (leukocyte adhesion deficiency, Chediak-Higashi syndrome, hyper-IgE syndrome, recurrent infection syndrome, and chronic granulomatous disease).27,28 All forms of neutropenia can result in oral mucosal pathology, primarily aphthous stomatitis and aggressive forms of gingivitis, which can be hyperplastic in nature (Fig. 10, Table 1).29,30
Treatment is varied and depends on the cause of the neutropenia but can include granulocyte-colony stimulating factor (G-CSF), prophylactic use of antibiotics and antifungals, interferon-gamma, allogeneic bone marrow transplantation, gene therapy, intravenous immunoglobulins, corticosteroids and other immunomodulatory or immunosuppressive therapies.
2.7 Plasma cell gingivitis
Plasma cell gingivitis (Fig. 11, Table 1) is a rare and benign condition, which is sometimes accompanied by cheilitis or glossitis ('plasma-cell gingivostomatitis'). It may represent a sub-type of plasma cell orofacial mucositis, which can be life threatening when it affects the airway and includes diverse entities such as Zoon's balanitis/vulvitis, which affect the genital mucosa, but may also be a separate disease entity. Other terms that have been used to describe this condition are idiopathic gingivostomatitis, atypical gingivostomatitis, allergic gingivostomatitis and plasmacytosis. The clinical presentation is that of erythematous mucosa with papillomatous, cobblestone, nodular, or granulomatous enlargement and it is histologically characterised by a dense plasma cell infiltrate.31 It may or may not involve the free gingival margin and can also be a localised lesion. Its aetiology is poorly understood but plasma cell gingivitis has been reported to be associated with hypersensitivity reactions to substances such as toothpaste and chewing gum ingredients, mint, pepper and cinnamon and in these cases resolution follows cessation of the offending allergen.32 However, beyond this there is currently no consensus on management of the disease.
3. Hereditary conditions
3.1 Hereditary gingival fibromatosis and idiopathic gingival hyperplasia
Hereditary gingival fibromatosis is a rare, mostly autosomal dominant disease, which can develop as an isolated disorder or a feature of a syndrome. It is a benign oral condition characterised by slow and progressive enlargement of both maxillary and mandibular attached gingiva. It is commonly associated with hypertrichosis (excess hair growth) as well as with epilepsy and intellectual disability.33A progressive fibrous enlargement of the gingiva is a feature of idiopathic hyperplasia/fibromatosis of the gingiva (Fig. 12, Table 1). Gingival fibromatosis may exist as an isolated abnormality or as part of a syndrome such as Laband syndrome.34 The exact cause of the disease is unknown; however, involvement of platelet-derived growth factor isoforms and its receptor as well as qualitative and quantitative differences in tumour growth factor-beta isoforms and receptor expression by fibroblasts have been suggested.35,36
Conclusion
In this article, the first part of this series of two papers, we have reviewed immune-mediated, autoinflammatory and hereditary gingival lesions, supported by epidemiological data, clinical descriptions and photographs. Several of these systemic conditions can have a similar clinical presentation to periodontitis and gingivitis or exacerbate existing disease. On the other hand, plaque-induced inflammation often worsens non plaque-induced conditions, which can make it more difficult to distinguish both. At the same time, dental practioners need to be aware that oral manifestations of systemic diseases can impair oral hygiene abilities in affected patients. Those lesions which are accompanied by pain, are non-resolving or show a morphology atypical of classic plaque-induced gingival inflammation, should encourage dental practioners to refer their patient to a specialist unit. A full discussion is included in part 2: cancer related, infective, and other causes of gingival pathology. Readers are therefore referred to this paper.37
References
Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol 2015; 15: 30-44.
Chi A C, Neville B W, Krayer J W, Gonsalves W C. Oral manifestations of systemic disease. Am Fam Physician 2010; 82: 1381-1388.
Chapple I L C, Mealey B L, Van Dyke T E et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018; 89(Suppl 1): S74S84.
Al-Abeedi F, Aldahish Y, Almotawa Z, Kujan O. The differential diagnosis of desquamative gingivitis: Review of the literature and clinical guide for dental undergraduates. J Int Oral Health 2015; 7(Suppl 1): 88-92.
Black M, Mignogna M D, Scully C. Number II. Pemphigus vulgaris. Oral Dis 2005; 11: 119-130.
Huang YH, Kuo CF, Chen YH, Yang YW. Incidence, mortality, and causes of death of patients with pemphigus in Taiwan: a nationwide population-based study. J Invest Dermatol 2012; 132: 92-97.
McMillan R, Taylor J, Shephard M et al. World Workshop on Oral Medicine VI: a systematic review of the treatment of mucocutaneous pemphigus vulgaris. Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 120: 132-142.e61.
Di Zenzo G, Carrozzo M, Chan L S. Urban legend series: mucous membrane pemphigoid. Oral Dis 2014; 20: 35-54.
Chan L S, Ahmed A R, Anhalt G J et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol 2002; 138: 370-379.
Scully C. PL6 Sub-epithelial vesiculobullous disorders: treatment now and on the horizon. Oral Dis 2006; 12(s1): 2.
Neff A G, Turner M, Mutasim D F. Treatment strategies in mucous membrane pemphigoid. Ther Clin Risk Manag 2008; 4: 617-626.
Chan L S. Ocular and oral mucous membrane pemphigoid (cicatricial pemphigoid). Clin Dermatol 2012; 30: 34-37.
Hasan S. Desquamative gingivitis - A clinical sign in mucous membrane pemphigoid: Report of a case and review of literature. J Pharm Bioallied Sci 2014; 6: 122-126.
Tilakaratne W M, Freysdottir J, Fortune F. Orofacial granulomatosis: review on aetiology and pathogenesis. J Oral Pathol Med 2008; 37: 191-195.
Alawi F. An Update on granulomatous diseases of the oral tissues. Dent Clin North Am 2013; 57: 657-671.
Critchlow W A, Chang D. Cheilitis granulomatosa: A review. Head Neck Pathol 2014; 8: 209-213.
Orphanet Report Series (Rare Disease Collection). 2018. Online information available at http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_decreasing_prevalence_or_cases.pdf (accessed May 2019).
Lankarani K B, Sivandzadeh G R, Hassanpour S. Oral manifestation in inflammatory bowel disease: A review. World J Gastroenterol 2013; 19: 8571-8579.
Mortada I, Leone A, Gerges Geagea A et al. Oral manifestations of inflammatory bowel disease. J Biol Regul Homeost Agents 2017; 31: 817-821.
Gupta S, Tripathi A K, Kumar V, Saimbi C S. Sarcoidosis: Oral and extra-oral manifestation. J Indian Soc Periodontol 2015; 19: 582-585.
Poate T W, Sharma R, Moutasim K A, Escudier M P, Warnakulasuriya S. Orofacial presentations of sarcoidosis - a case series and review of the literature. Br Dent J 2008; 205: 437-442.
Hanisch M, Fröhlich L F, Kleinheinz J. Gingival hyperplasia as first sign of recurrence of granulomatosis with polyangiitis (Wegener's granulomatosis): case report and review of the literature. BMC Oral Health 2017; 17: 33.
Alrashdan M S, Cirillo N, McCullough M. Oral lichen planus: a literature review and update. Arch Dermatol Res 2016; 308: 539-551.
McCartan B E, Healy C M. The reported prevalence of oral lichen planus: a review and critique. J Oral Pathol Med 2008; 37: 447-453.
Gupta S, Jawanda M K. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol 2015; 60: 222-229.
Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 2006; 6: 173-182.
Schwartzberg L S. Neutropenia: Etiology and Pathogenesis. Clin Cornerstone 2006; 8: S5-S11.
Capsoni F, Sarzi-Puttini P, Zanella A. Primary and secondary autoimmune neutropenia. Arthritis Res Ther 2005; 7: 208-214.
Tirali R E, Yalcinkaya Erdemci Z, Cehreli S B. Oral findings and clinical implications of patients with congenital neutropenia: a literature review. Turk J Pediatr 2013; 55: 241-245.
Park M S, Tenenbaum H C, Dror Y, Gloguaer M. Oral health comparison between children with neutropenia and healthy controls. Special Care Dent 2014; 34: 12-18.
Galvin S, Bowe C, O'Regan E M, Conlon N, Flint S R, Healy C M. Circumorificial plasmacytosis/plasma cell orificial mucositis: a case series and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol 2016; 122: e77-81.
Janam P, Nayar B R, Mohan R, Suchitra A. Plasma cell gingivitis associated with cheilitis: A diagnostic dilemma! J Indian Soc Periodontol 2012; 16: 115-119.
Dhadse P V, Yeltiwar R K, Pandilwar P K, Gosavi S R. Hereditary gingival fibromatosis. J Indian Soc Periodontol 2012; 16: 606-609.
Cekmez F, Pirgon O, Tanju I A. Idiopathic gingival hyperplasia. Int J Biomed Sci 2009; 5: 198-200.
Wright H J, Chapple I L, Matthews J B. TGF-beta isoforms and TGF-beta receptors in drug-induced and hereditary gingival overgrowth. J Oral Pathol Med 2001; 30: 281-289.
Wright H J, Chapple I L, Cooper P, Matthews J B. Platelet-derived growth factor (PDGF) isoform and PDGF receptor expression in drug-induced gingival overgrowth and hereditary gingival fibrosis. Oral Dis 2006; 12: 315-323.
Hirschfeld J, Higham J, Blair F, Richards A, Chapple I. Systemic disease or periodontal disease? Distinguishing causes of gingival inflammation: a guide for dental practicioners. Part 2: cancer related, infective, and other causes of gingivial pathology. Br Dent J 2019; In press.
Acknowledgements
The authors would like to thank Mr Mutahir Rahman at the Department of Periodontology of the Birmingham Dental School and Hospital for his permission to use clinical photographs. We further thank our patients for their consent to utilise their clinical photographs for this publication.
Author information
Authors and Affiliations
Contributions
JH (Josefine Hirschfeld) took the lead in writing and finalising the manuscript. JH (Jon Higham), DC and JH drafted the manuscript and gathered demographic data for the table; FB, AR and ILC provided guidance and clinical photographs. All authors provided critical feedback.
Corresponding author
Rights and permissions
About this article
Cite this article
Hirschfeld, J., Higham, J., Chatzistavrianou, D. et al. Systemic disease or periodontal disease? Distinguishing causes of gingival inflammation: a guide for dental practitioners. Part 1: immune-mediated, autoinflammatory, and hereditary lesions. Br Dent J 227, 961–966 (2019). https://doi.org/10.1038/s41415-019-1050-8
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41415-019-1050-8