Abstract
Risk factors for severe SARS-Cov-2 infection course are poorly described in children following hematopoietic cell transplantation (HCT). In this international study, we analyzed factors associated with a severe course (intensive care unit (ICU) admission and/or mortality) in post-HCT children. Eighty-nine children (58% male; median age 9 years (min-max 1–18)) who received an allogeneic (85; 96%) or an autologous (4; 4%) HCT were reported from 28 centers (18 countries). Median time from HCT to SARS-Cov-2 infection was 7 months (min-max 0–181). The most common clinical manifestations included fever (37; 42%) and cough (26; 29%); 37 (42%) were asymptomatic. Nine (10%) children following allo-HCT required ICU care. Seven children (8%) following allo-HCT, died at a median of 22 days after SARS-Cov-2 diagnosis. In a univariate analysis, the probability of a severe disease course was higher in allo-HCT children with chronic GVHD, non-malignant disease, immune suppressive treatment (specifically, mycophenolate), moderate immunodeficiency score, low Lansky score, fever, cough, coinfection, pulmonary radiological findings, and high C-reactive protein. In conclusion, SARS-Cov-2 infection in children following HCT was frequently asymptomatic. Despite this, 10% needed ICU admission and 8% died in our cohort. Certain HCT, underlying disease, and SARS-Cov-2 related factors were associated with a severe disease course.
Similar content being viewed by others
Introduction
It has been more than 2 years since the World Health Organization announced a global coronavirus disease (COVID-19) pandemic. Within this pandemic, the proportion of children among all COVID-19 cases is increasing [1]. In immunocompetent children, COVID-19 is usually a mild disease with low rates of both intensive care unit (ICU) admissions (0.8%) and of mortality (<0.1%) [2]. However, children with underlying diseases, including those with immune deficiency and cancer, are at higher risk of developing severe COVID-19 than healthy children [3,4,5].
Data on SARS-Cov-2 infection in hematopoietic cell transplantation (HCT) recipients were prospectively collected by the European Society for Blood and Marrow Transplantation (EBMT) and the Spanish Group of Hematopoietic Stem Cell Transplantation (GETH). Previous analysis, including cases diagnosed before August 2020, reported an ~30% mortality in HCT recipients following COVID-19 during the first wave [6], with a better outcome in children. Factors associated with a severe clinical course in children with SARS-Cov-2 following HCT are, however, poorly described. In this report, we present clinical and outcome data on SARS-Cov-2 infection in children from this registry.
Subjects and methods
Data concerning patients (both children and adults) who developed SARS-Cov-2 following allogeneic or autologous HCT were prospectively collected by the EBMT [6]. Invitation to participate in the study was distributed among the EBMT centers. All patients give informed consent for their data to be included in the EBMT registry. Cases were identified through voluntary reporting. Background HCT-related data routinely reported by all EBMT centers were collected from the EBMT registry, including patient demographics, presence of comorbidities, primary diagnosis, status, HCT type, donor type and conditioning regimen. These data were combined with those relating to the SARS-Cov-2 from specially designed case record forms providing data at diagnosis and 6-weeks follow-up. The forms were updated based on the clinical and management developments reached during the pandemic. A parallel data collection with forms written in Spanish was used to collect data from Spain by GETH [6].
The SARS-Cov-2 information included clinical manifestation, need for hospitalization, need for ICU admission, treatment, and outcome, as well as potential risk factors for a severe disease course at the time of SARS-Cov-2 infection. We defined severe disease as ICU admission during the SARS-Cov-2 infection or death within two months of SARS-Cov-2 diagnosis. Immunodeficiency scoring index was defined as described [6, 7].
We present data on children aged 0–18 years with a SARS-Cov-2 diagnosis reported to the EBMT/GETH survey before December 16, 2021. All children were diagnosed by SARS-CoV-2 positive PCR on respiratory tract samples. Our primary objective was to describe SARS-Cov-2 characteristics and outcomes. The secondary objective was to characterize factors associated with severe disease.
Statistical analysis
The main characteristics of patients were reported by descriptive statistics. Continuous data were presented as median, minimum, and maximum values. Categorical data were presented as numbers and percentage of total. The probability of a severe course was assessed using Kaplan-Meier estimator and compared between groups using log-rank tests and calculating cumulative incidence and hazard ratio. To identify factors associated with severe disease, a univariate Cox regression model was used. The following factors were analyzed: demography, underlying diagnosis, HCT source and conditioning; at the time of SARS-CoV-2 infection: underlying disease status, Lansky score, presence of comorbidities, steroid therapy, neutrophil and lymphocyte counts, presence of acute or chronic graft versus host disease (GVHD), and other immunosuppressive therapy medications within 2 months of SARS-Cov-2 infection onset.
A bilateral p value <0.05 was considered as statistically significant. All analyses were performed using the statistical software SAS v.9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Study population (Table 1)
Eighty-nine children were reported from 28 centers, in 18 countries. Timing of cases diagnosis is presented in Supplementary Fig. 1. Fifty-two (58%) were male; median age was 9 years min–max 1–18). Eighty-five (96%) had undergone an allogeneic and 4 (4%) and autologous HCT. The median time from the most recent HCT to SARS-Cov-2 infection was 7 months (min-max 0–181). The most common underlying diseases were acute leukemia (46; 52%), and inherited disorders (15; 17%). Twenty-one (24%) children had comorbidities; among them, seven (8%) had lung pathology; five (6%) had hypertension (one of them with dyslipidemia), three (3%) had cardiovascular comorbidities, and six (7%) children had diabetes, obesity, secondary hemosiderosis, dyslipidemia, and cerebral blood vessels stenosis. At the time of SARS-Cov-2 infection, 19 (22%) and 12 (16%) of children had acute and chronic GVHD, respectively. None was vaccinated.
SARS-CoV-2 infection manifestations and treatment
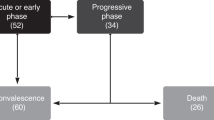
Clinical and laboratory parameters are presented in Table 2. The median time from symptoms onset to SARS-Cov-2 infection diagnosis in 40 children symptomatic at the time of diagnosis was 2 days (min-max 0-40). Thirty-seven (42%) children were asymptomatic during infection course. The most common clinical manifestations included fever, cough, and upper respiratory tract symptoms, including rhinorrhea, sinusitis, otitis, or pharyngitis. Twenty-four children (27%) had evidence of coinfections, mainly viral (Supplementary Table 1). Forty-nine (55%) children were hospitalized; 21 (43%) due to COVID-19. Nine (10%) children required oxygen to maintain oxygen saturation above 92%; three (3%) and five (6%) children required non-invasive and invasive ventilation, respectively. Eighteen out of 62 cases (29%) with lung radiology performed had abnormal pulmonary radiological findings. Treatments are summarized in Supplementary Table 2.
Children with a severe course (Tables 1, 2, 3)
Nine (10%) children required ICU care within a median 10.5 days (min-max -5–29) following a SARS-Cov-2 diagnosis. One of these children was in intensive care for five days prior to the diagnosis. Initially hospitalized in the ICU for seizures that improved on treatment, his consciousness decreased two days later, and he developed fever. Brain MRI revealed meningoencephalitis. SARS-Cov-2 PCR, taken for the first time five days after ICU admission, was positive. No other infections were identified, so SARS-Cov-2 infection possibly contributed to this patient’s neurological symptoms and ICU admission.
All children hospitalized in the ICU were neutropenic at the time of SARS-Cov-2 diagnosis and were receiving IST as a part of their transplantation management (not for COVID-19 treatment); eight of nine were lymphocytopenic. Three children required non-invasive and five invasive ventilation. Median ICU hospitalization duration was 10.5 days (min-max; 3–38); five of these children died after a median 13 days (min-max, 7–38) from ICU admission.
Seven (8%) children (five males) who developed SARS-Cov-2 infection at a median of 2 months (min-max; 0–147) after HCT died (Tables 1 and 2). All had undergone allo-HCT, all received immune suppression at the time of SARS-Cov-2 diagnosis. The median time from the SARS-Cov-2 diagnosis to death was 22 days (min-max; 5–54). Six had persistent positive SARS-CoV-2 PCR at the time of death. In five children, there was evidence of bacterial, viral, or fungal coinfections that could contribute to death (Supplementary Table 1). In two children, who died one and 22 days after SARS-Cov-2-I diagnosis, the presumed main causes of death were relapse and non-engraftment, respectively. One child died suddenly 54 days after SARS-Cov-2 infection diagnosis. None of children who died received medications listed in Supplementary Table 2.
In the univariate analysis, the probability of a severe disease course was higher in the following children after allo-HCT: non-malignant underlying disease, chronic GVHD, exposure to IST, and specifically to mycophenolic acid (MMF), moderate risk based on immunodeficiency scoring index (vs. low risk), presence of fever, cough, coinfection, and pulmonary radiological findings. C-reactive protein (CRP) levels were significantly higher; and Lansky score was significantly lower at the time of SARS-Cov-2 infection in children with a severe course (Supplementary Figs. 2 and 3). Due to the low number of events, it was not possible to perform the multivariate analysis.
We compared the group of children with malignant and non-malignant diseases, and we found that in the group of children with non-malignant diseases there was a higher proportion of T-cell depletion (26/31; 84% vs. 24/56; 43%, p = 0.0002) and comorbidities (9/33; 27% vs. 5/56; 9%; p = 0.02). There were no significant differences in the demography, conditioning, immune suppressive treatment, proportion of acute or chronic GVHD, coinfections, neutropenia or lymphocytopenia.
Discussion
In this quite large series of pediatric HCT recipients with SARS-Cov-2 infection, we present the clinical picture and report the factors related to the underlying disease and HCT course (chronic GVHD, non-malignant disease, exposure to IST, and specifically to MMF, moderate risk based on immunodeficiency scoring index (vs. low risk), lower Lansky score) and to SARS-Cov-2-I course (fever, cough, radiological pulmonary findings, co-infections, high CRP) that were associated with severe disease. Our finding that chronic GVHD and ongoing immune suppression predispose to a severe disease course has an important practical implication. Recent data in a general population demonstrate growing numbers of children infected with SARS-Cov-2, as well as a growing proportion of them requiring hospitalization [8]. Vaccination, recently approved from age 6 months, is an important protective measure. Vaccine efficacy, however, is impaired in patients with chronic GVHD and those receiving IST [9]. The fact that such children are at increased risk of severe disease course and mortality underscores the importance of additional precautions—among them, vaccination of family members, masks, and pre-exposure prophylaxis for eligible patients [10].
There are few treatment options for pediatric patients with SARS-Cov-2, especially for non-hospitalized children, with limited data on their efficacy and controversy over their recommended use as the course of COVID-19 is usually mild, even in children with malignancies. Children with mild-to-moderate COVID-19 weighing at least 3.5 kg who are at high risk for progression to severe disease, including hospitalization or death, can be treated with remdesivir, as its indications for its use were expanded by the FDA [11], and our study helps identifying post-HCT children who are at higher risk for adverse outcomes. More severely ill children, such as those who require conventional oxygen, oxygen through high-flow device or non-invasive ventilation, shall receive treatment with remdesivir with or without dexamethasone, as recommended by the NIH [12]. These drugs were, however, rarely used in patients reported to our study, and none of the children with severe outcome was treated according to the current NIH guidelines.
Comparison of data in our study to other pediatric multicenter studies and to the EBMT/GETH study [6] is presented in Table 4. To identify relevant studies, we searched PubMed at the end of June 2022 for the following items: ‘((COVID OR SARS-CoV 2) AND (children OR adolescents OR pediatric)) AND ((stem cell transplantation) OR (bone marrow transplantation)). The prospective EBMT/GETH study summarized data of 382 patients post-HCT, among them 32 children, reported until July 2020 [6]. Survival was presented separately for the pediatric and adult populations, whereas clinical manifestations and mortality risk factors were for the whole study population. Our current research presents data on children post-HCT reported to the EBMT registry from March 2020 until the end of 2021, including the 32 children from the EBMT/GETH study. Comparison of the current pediatric data with the overall EBMT/GETH cohort including predominantly adult population, reveals several differences. Amongst them are a higher proportion of asymptomatic infections (42% vs. 9%), and a lower proportion of febrile patients and those requiring oxygen to maintain saturation (43 vs. 65% and 11 vs. 35%, respectively) in the current study; the difference between children and adults is probably even bigger, as data on 32 children is included in the EBMT/GETH report [6]. Ten percent of children in our study were admitted to the ICU and 8% died. Mortality rates in the EBMT/GETH study’s overall patient population were 23% and 28% in allogeneic and autologous HCT recipients, respectively; survival in children was significantly higher. It should be noted that later in the pandemic, with improved COVID-19 management and effective medications and vaccines, and variant changes, lower mortality rates were observed in adults following HCT (PL, submitted to publication). Similarly, CIBMTR study including 29 children and 289 adults reported a 22% and 19% mortality rates in allogeneic and autologous HCT recipients, respectively [13]. Another CIBMTR study that summarized data on 135 children following allo-HCT and 32 children following auto-HCT, reported 6% mortality rate and 4% rate of mechanical ventilation [14]. A multicenter Brazilian study found higher mortality rates in children post-HCT—5/24 (21%) [15]—with mortality rates in adults post-HCT comparable with those in the EBMT/GETH study (21/62, 34%). A summary of 54 post-allogeneic pediatric HCT recipients with COVID-19 described in case series and case reports yields figures of 7/27 (26%) ICU admissions and 5/54 (9.3%) mortality [16]. Additional reports on SARS-Cov-2 in children with cancer, including some HCT recipients, found an 0 to 18% rate of ICU admission, and 0 to 5% mortality rate [17,18,19,20,21,22,23]. The rates of the ICU admission and mortality in the Pediatric Oncology COVID-19 Case Report (POCC) registry at the pre-Omicron time period (1584 children registered up to January 2022) were 5% and 7%, respectively, in children with solid tumors; and 8% and 3% in children with hematological malignancies [24]. In another registry, Global Registry of COVID in Childhood cancer, (1814 children registered at November 2022), 8.5% were admitted to the ICU and 3.6% died [25].
The proportion of patients in our study requiring oxygen (11%), as well as in the CIBMTR pediatric study (10%) was considerably lower than the 32% of 87 pediatric cancer patients reported in a review of 20 studies [14, 23]. This is most likely due to the large number of asymptomatic infections in our series.
The proportion of children post-HCT with a severe COVID-19 course is markedly higher than in the general pediatric population. The Centers for Disease Control and Prevention 2021 Morbidity and Mortality Weekly Report (MMWR) reports that 0.8% of 0- to 17-year-olds in the US need ICU care, with a mortality <0.1% [2]. We found a higher proportion of children with radiological evidence of pneumonia (29%) than that reported among SARS-Cov-2 -infected children who sought emergency care in a nationwide Israeli study [26]. These high rates of severe disease due to respiratory viruses in HCT children are not unique to SARS-Cov-2. Similar rates of ICU admission (9–19%) and mortality (1–10%) were reported in children post-HCT infected with other respiratory viruses, such as influenza and respiratory syncytial virus [27,28,29].
Risk factors for severe COVID-19 disease post HCT are poorly defined in children. Consistent with our data, low performance status was associated with inferior outcomes in the overall EBMT/GETH study cohort, and ongoing immunosuppression increased the risk of ICU admission in allogeneic HCT recipients [6]. In a pediatric CIBMTR study, 45-day survival was lower among recipients transplanted in the centers outside the US and those transplanted between 2014 and 2020 versus 2000–2013 [14]. In a Brazilian study of 62 adults and 24 children, Eastern Cooperative Oncology Group Performance Status and severity of clinical presentation were associated with mortality in the overall study population, and male gender was associated with mortality in children [15]. In contrast to our data, other studies also reported a higher proportion of males among immunocompromised children with severe COVID-19 [15, 17, 19]. Higher risk of severe clinical course in children with non-malignant diseases was not reported previously. Higher rate of T-cell depletion and comorbidities in these children could probably contribute to this, and shall be assessed in the multivariate analysis. In a multicentre Italian study, infections occurring early (<60 days) after the diagnosis or after SCT were associated with higher risk of moderate/severe/critical disease [30]. We, however, did not observe higher rate of severe disease course during the early pre-HCT period. Literature data on factors associated with a severe disease course in immunocompromised children are presented in Table 4. It should be mentioned that mortality in our study was multifactorial, as five out of seven children who died had coinfections that could contribute to adverse outcome.
The median period from HCT to SARS-Cov-2 infection (6 months) in our study is far shorter than that in the overall EBMT/GETH study cohort (17.9 months) and in the CIBMTR reports [6, 13, 14]. This difference is probably attributable to the mild course of pediatric COVID-19 and a high proportion of asymptomatic infections, that could mean that many pediatric SARS-Cov-2 infections occurring late after HCT go undiagnosed or unreported to the HCT center, especially if more time has elapsed since HCT.
Our study has limitations. First, most of the patients included are from the pandemic’s early waves, with clinical presentation and outcome sometimes differing with subsequent variants. The patients also represent a large and non-vaccinated cohort and the more widespread use of vaccines also in children both before and after HCT might ameliorate the seriousness of COVID-19 further. Second, our sample size, and, fortunately, low rate of severe disease, precludes multivariate analysis of risk factors, and identification of independent risk factors for a severe disease course in children. Univariate analysis revealed several risk factors, but these results should be interpreted with caution due to small numbers. Third, there are data on treatment only for a minority of children, and we cannot conclude on the correlation between treatment and disease outcome. Forth, cases were not consecutively collected but were based on voluntary reporting and hence are subject to reporter bias.
Our study also, however, has important strengths. It represents a large cohort of children infected with SARS-Cov-2 following HCT. Its clinical, laboratory and outcome data are detailed. In addition, it is the first study that has enabled risk factor analysis for a severe clinical course in children with SARS-Cov-2 post-HCT, with potential clinical implications.
To conclude, SARS-Cov-2 following HCT in children was frequently asymptomatic. The rates of ICU admission (10%) and mortality (8%) was lower than in immunocompromised adults; they are, however, higher than those reported in immunocompetent children, and comparable to these risks reported for post-HCT children infected with other respiratory viruses. HCT and underlying disease related factors (non-malignant disease, chronic GVHD, IST and specifically MMF exposure, moderate risk based on immunodeficiency scoring index (vs. low risk), low Lansky score), as well as SARS-Cov-2 related factors (fever, cough, pulmonary radiological findings, high CRP, and coinfections) are associated with ICU admission and mortality in a univariate analysis and should be confirmed in a bigger study.
The partial results of this study were presented as a poster in the American Society of Hematology Conference 2021.
References
Pediatrics AAo. Children and COVID-19: State-Level Data Report, 2022. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/
Leidman E, Duca LM, Omura JD, Proia K, Stephens JW, Sauber-Schatz EK. COVID-19 trends among persons aged 0-24 years—United States, March 1–December 12, 2020. Morb Mortal Wkly Rep. 2021;70:88–94.
Gotzinger F, Santiago-Garcia B, Noguera-Julian A, Lanaspa M, Lancella L, Calo Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–61.
Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174:868–73.
Akcay N, Kihtir HS, Durak C, Kendirli T, Havan M, Kockuzu E, et al. Mortality risk factors among critically Ill children with acute COVID-19 in PICUs: a multicenter study from turkish pediatric critical COVID-19 and MIS-C Study Group. Pediatr Infect Dis J. 2022;41:742–50.
Ljungman P, de la Camara R, Mikulska M, Tridello G, Aguado B, Zahrani MA, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021;35:2885–94.
Shah DP, Ghantoji SS, Ariza-Heredia EJ, Shah JN, El Taoum KK, Shah PK, et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood. 2014;123:3263–8.
Marks KJ, Whitaker M, Agathis NT, Anglin O, Milucky J, Patel K, et al. Hospitalization of infants and children aged 0-4 years with laboratory-confirmed COVID-19 - COVID-NET, 14 states, March 2020-February 2022. Morb Mortal Wkly Rep. 2022;71:429–36.
Shem-Tov N, Yerushalmi R, Danylesko I, Litachevsky V, Levy I, Olmer L, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in haematopoietic stem cell transplantation recipients. Br J Haematol. 2022;196:884–91.
Tixagevimab and Cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19. JAMA 2022; 327: 384–5.
FDA. FDA takes actions to expand use of treatment for outpatients with mild-to-moderate COVID-19, 2022. https://www.fda.gov/news-events/press-announcements/fda-takes-actions-expand-use-treatment-outpatients-mild-moderate-covid-19.
Health AowotNIo. NIH. COVID-19 Treatment Guidelines. 2022. An official website of the National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/tables/therapeutic-management-of-hospitalized-children/
Sharma A, Bhatt NS, St Martin A, Abid MB, Bloomquist J, Chemaly RF, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8:e185–e193.
Bhatt NS, Sharma A, Martin AS, Abid MB, Brown VI, Diaz MA, et al. Clinical characteristics and outcomes of COVID-19 in pediatric and early adolescent and young adult haematopoietic stem cell transplant recipients: a cohort study. Transplant Cell Ther. 2022;28:696.e1–696.e7.
Daudt LE, Corso MCM, Kerbauy MN, de Assis L, Rechenmacher C, Colturato I, et al. COVID-19 in HSCT recipients: a collaborative study of the Brazilian Society of Marrow Transplantation (SBTMO). Bone Marrow Transplant. 2022;57:453–9.
Bailey AJM, Kirkham AM, Monaghan M, Shorr R, Buchan CA, Bredeson C, et al. A portrait of SARS-CoV-2 infection in patients undergoing hematopoietic cell transplantation: a systematic review of the literature. Curr Oncol. 2022;29:337–49.
Faura A, Rives S, Lassaletta A, Sebastian E, Madero L, Huerta J, et al. Initial report on Spanish pediatric oncologic, hematologic, and post stem cell transplantation patients during SARS-CoV-2 pandemic. Pediatr Blood Cancer. 2020;67:e28557.
Kebudi R, Kurucu N, Tugcu D, Hacisalihoglu S, Fisgin T, Ocak S, et al. COVID-19 infection in children with cancer and stem cell transplant recipients in Turkey: a nationwide study. Pediatr Blood Cancer. 2021;68:e28915.
Madhusoodhan PP, Pierro J, Musante J, Kothari P, Gampel B, Appel B, et al. Characterization of COVID-19 disease in pediatric oncology patients: the New York-New Jersey regional experience. Pediatr Blood Cancer. 2021;68:e28843.
Rouger-Gaudichon J, Thebault E, Felix A, Phulpin A, Paillard C, Alimi A, et al. Impact of the first wave of COVID-19 on pediatric oncology and hematology: a report from the French Society of Pediatric Oncology. Cancers. 2020;12:3398.
Haeusler GM, Ammann RA, Carlesse F, Groll AH, Averbuch D, Castagnola E, et al. SARS-CoV-2 in children with cancer or after haematopoietic stem cell transplant: an analysis of 131 patients. Eur J Cancer. 2021;159:78–86.
Mukkada S, Bhakta N, Chantada GL, Chen Y, Vedaraju Y, Faughnan L, et al. Global characteristics and outcomes of SARS-CoV-2 infection in children and adolescents with cancer (GRCCC): a cohort study. Lancet Oncol. 2021;22:1416–26.
Meena JP, Kumar Gupta A, Tanwar P, Ram Jat K, Mohan Pandey R, Seth R. Clinical presentations and outcomes of children with cancer and COVID-19: a systematic review. Pediatr Blood Cancer. 2021;68:e29005.
Johnston EEMI, Davis ES, Araya B, Richman J, Brackett J, Dickens D, et al. The Pediatric Oncology COVID-19 Case (POCC) Report. April 29, 2020. https://www.uab.edu/medicine/icos/images/POCC_Report/Bi-weekly_POCC_Report/Report.1.26.22.pdf. accessed 31 Oct 2022.
Hospital SJCsR. Global Registry of COVID-19 in Childhood Cancer, 2022. https://app.powerbi.com/view?r=eyJrIjoiNGQ3NDAwZDItYjRjNi00MjNhLWE2NTMtNmFjNmU1YTgzZDMwIiwidCI6IjIyMzQwZmE4LTkyMjYtNDg3MS1iNjc3LWQzYjNlMzc3YWY3MiIsImMiOjN9. accessed 31 Oct 2022.
Ben-Shimol S, Livni G, Megged O, Greenberg D, Danino D, Youngster I, et al. COVID-19 in a subset of hospitalized children in Israel. J Pediatr Infect Dis Soc. 2021;10:757–65.
Atalla E, Kalligeros M, Mylona EK, Tsikala-Vafea M, Shehadeh F, Georgakas J, et al. Impact of influenza infection among adult and pediatric populations with hematologic malignancy and hematopoietic stem cell transplant: a systematic review and meta-analysis. Clin Ther. 2021;43:e66–e85.
Hutspardol S, Essa M, Richardson S, Schechter T, Ali M, Krueger J, et al. Significant transplantation-related mortality from respiratory virus infections within the first one hundred days in children after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:1802–7.
Rowan CM, Gertz SJ, Zinter MS, Moffet J, Bajwa RPS, Barnum JL, et al. A multicenter investigation of respiratory syncytial viral infection in children with hematopoietic cell transplantation. Transpl Infect Dis. 2018;20:e12882.
Zama D, Baccelli F, Colombini A, Contino A, Calore E, Petris MG, et al. Favorable outcome of SARS-CoV-2 infection in pediatric hematology oncology patients during the second and third pandemic waves in Italy: a multicenter analysis from the Infectious Diseases Working Group of the Associazione Italiana di Ematologia e Oncologia Pediatrica (AIEOP). Ann Hematol. 2022;101:1843–51.
Acknowledgements
Cristina Belendez Bieler, Hospital Univ.Materno Infantil Gregorio Marañon, Madrid, Spain; Antonio Campos, Inst. Português de Oncologia do Porto, Porto, Portugal; Matthew Collin, Adult HSCT unit, Newcastle_Tyne, UK; Josu de la Fuente, Division of Paediatrics, London, UK; Marta Gonzalez Vicent, Niño Jesus Children’s Hospital, Madrid, Spain; Krzysztof Kalwak, Fundacja “Na Ratunek Dzieciom z Choroba Nowotworowa”, Wroclaw, Poland; Anjum Khan, Yorkshire Blood & Marrow Transplant Programme, Leeds, UK; Ulker Kocak, Gazi University School of Medicine, Ankara, Turkey; Régis Peffault de Latour, Hopital St. Louis, Paris, France; Paul G. Schlegel, University Children’s Hospital, Wuerzburg, Germany; Joan Hendrik Veelken, Leiden University Hospital, Leiden, Netherlands;
Author information
Authors and Affiliations
Contributions
Conceptualization and design: DA, PL. Collection and assembly of data: NK. Data analysis: DA, GT. All authors helped with data interpretation, critical review of manuscript, appropriate revisions and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
RC: advisor for AstraZeneca in EVUSHELD pre-exposure prophylaxis for SARS-CoV-2. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Averbuch, D., de la Camara, R., Tridello, G. et al. Risk factors for a severe disease course in children with SARS-COV-2 infection following hematopoietic cell transplantation in the pre-Omicron period: a prospective multinational Infectious Disease Working Party from the European Society for Blood and Marrow Transplantation group (EBMT) and the Spanish Group of Hematopoietic Stem Cell Transplantation (GETH) study. Bone Marrow Transplant 58, 558–566 (2023). https://doi.org/10.1038/s41409-023-01941-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-01941-5