Abstract
Autoimmune diseases (ADs) represent a heterogenous group of complex diseases with increasing incidence in Western countries and are a major cause of morbidity. Hematopoietic stem cell transplantation (HSCT) has evolved over the last 25 years as a specific treatment for patients with severe ADs, through eradication of the pathogenic immunologic memory and profound immune renewal. HSCT for ADs is recently facing a unique developmental phase across transplant centers. This review provides a comprehensive overview of the recent evidence and developments in the area, including fundamentals of preclinical research, clinical studies in neurologic, rheumatologic and gastroenterologic diseases, which represent major indications at present, along with evidence of HSCT for rarer indications. Moreover, we describe the interwoven challenges of delivering more advanced cellular therapies, exploiting mesenchymal stem cells, regulatory T cells and potentially CAR-T cell therapies, in patients affected by ADs. Overall, we discuss past and current indications, efficacy, associated risks and benefits, and future directions of HSCT and advanced cellular therapies in the treatment of severe/refractory ADs, integrating the available literature with European Society for Blood and Marrow Transplantation (EBMT) registry data.
Similar content being viewed by others
Introduction
Over the last 25 years, hematopoietic stem cell transplantation (HSCT) has been increasingly used to treat patients affected by severe and refractory autoimmune diseases (ADs) [1]. The majority of such patients have chronic diseases, which impact on quality-of-life and can shorten life expectancy but are rarely life-threatening in the short-term. Autologous and allogeneic HSCT are performed in this population after a careful balance of benefits and risks, and consideration of alternative treatment options [2, 3], with the aim to ablate the aberrant immune system and reconstitute one more tolerant to self-antigens, inducing a complete and stable remission from disease activity [4].
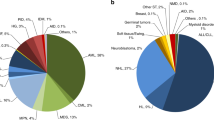
Recent evidence base, as developed across the international scientific research communities, explored HSCT as single one-off procedure to treat a variety of severe Ads [5]. Multiple sclerosis (MS) and systemic sclerosis (SSc) cover around 80% of transplants performed for ADs (Figs. 1, 2), where HSCT has become an integral and standard-of-care part of treatment algorithms [6, 7].
Autologous transplants for ADs reported to the EBMT-ADWP data registry, from 1994 through 2021. Major indications for autologous HSCT are multiple sclerosis, systemic sclerosis, and Crohn’s disease. AD autoimmune disease, ADWP Autoimmune Diseases Working Party, EBMT European Society for Blood and Marrow Transplantation, HSCT hematopoietic stem cell transplantation, IDD insulin-dependent diabetes mellitus, MS multiple sclerosis, SSc systemic sclerosis, SLE systemic lupus erythematosus.
Number of autologous HSCT reported per year and indication, from 1994 through 2021 (n = 3502). AD autoimmune disease, ADWP Autoimmune Diseases Working Party, EBMT European Society for Blood and Marrow Transplantation, HSCT hematopoietic stem cell transplantation, IDD insulin-dependent diabetes mellitus, MS multiple sclerosis, SSc systemic sclerosis, SLE systemic lupus erythematosus.
An analysis of trends across all ADs over the first 20 years of activity reflected the growth across indications, improvements in outcomes related to experience and center size, with some support for benefit of accreditation and economic factors [8]. A multidisciplinary approach is key in this field. The EBMT Autoimmune Diseases Working Party (ADWP) is central to bringing together HSCT and disease specialist communities. The AD section of the EBMT Registry is the largest database of its kind worldwide, reporting more than 3700 transplants.
In this review, we discuss past and current indications, efficacy, associated risks and benefits, and future directions of HSCT and advanced cellular therapies in the treatment of severe/refractory ADs, integrating the available literature with EBMT registry data. Moreover, we offer a thorough insight into the pathophysiology and management of ADs, and provide a general guidance on the entire transplant process, from the biological rationale and preclinical models to clinical results and recommendations.
Biology of immune cells during aging, autoimmunity, and stem cell transplantation
Despite the clinical heterogeneity of ADs, many of them share common genetic and molecular patterns leading to a loss of tolerance and chronic immunologic self-reactivity [9]. On a cellular level, this is reflected by profound age-associated changes of B cells, such as decreased B-cell differentiation in the bone marrow [10], redistribution of B-cell subsets in the periphery with a significant increase of proinflammatory (CD19+CD27−IgD−) double-negative B cells [11], and decreased expression of molecules involved in immunoglobulin class switching and somatic hypermutation, leading to decreased repertoire diversity and alterations of antibody responses [12]. Similarly, T cells undergo profound alterations during the aging process, ultimately resulting in immunosenescence [13], most importantly associated with thymic involution with reduced T-cell receptor repertoire diversity [14, 15]. In contrast, antibody-secreting memory plasma cells, which are most recognizable for their longevity [16], are stably maintained during aging [17], as their survival is controlled by dedicated survival niches in the bone marrow [18]. Importantly, long-lived plasma cells not only provide protective humoral immunity, they also contribute to the chronicity of various ADs by the persistent secretion of pathogenic antibodies [19], and thus, resemble novel therapeutic targets in autoimmunity [20]. The effect of HSCT on immunologic renewal has been the focus of many mechanistic studies. They collectively demonstrated that many of the age- or autoimmune-mediated immune disturbances may improve or even resolve [5]. For example, immune reconstitution studies indicated a predominant repopulation of CD27−IgD+ naïve B cells [21, 22], a stable output of recent thymic emigrants [23, 24], leading to a re-diversification of the receptor repertoire, and recurrence of both regulatory B and T cells in various Ads [25,26,27,28].
General concepts and process of HSCT for ADs
The initial concept of using HSCT as a means of controlling severe ADs was based on animal experiments and case reports showing long remission of ‘coincidental’ AD alongside standard hematological HSCT indications [29, 30]. Autologous HSCT is largely adopted to provide rapid and sustained hematopoietic recovery, supporting regimens of high-dose cytotoxic chemotherapy with lymphodepleting serotherapy (most commonly anti-thymocyte globulin, ATG). Nowadays, due to the less invasive collection methods and more rapid resolution of neutropenia and thrombocytopenia, peripheral blood stem cells (PBSCs) have largely replaced bone marrow as source, using cryopreservation techniques. After mobilization with granulocyte-colony stimulating factor (G-CSF) with or without cyclophosphamide (Cy), the graft is collected by leukapheresis and may be manipulated to enrich hematopoietic stem cells (CD34 selection) [31] and remove immune cells (ex vivo T cell depletion), potentially removing self-reactive lymphocytes [5].
Different conditioning regimens may be administered before the reinfusion of the cells in patients affected by ADs. The overall intensity of the conditioning regimen [32] varies greatly according to disease indication, study protocol and center preference. The optimal intensity of the conditioning is still to be defined in ADs, due to wide clinical variability of patients and lack of direct comparative trials. The final intensity of the immunosuppression is affected by many factors (type of conditioning and inclusion/dosage of serotherapy, mobilization chemotherapy, CD34 selection as graft manipulation, and prior treatments), leading to consider more ‘treatment intensity’ rather than just ‘conditioning intensity’ in the context of Ads [2, 5]. Reduced intensity [33] conditioning regimen significantly reduce the risk of treatment-related morbidity such as infections, infertility, organ damage, as well as treatment-related mortality (TRM). Indeed, there was chronological improvement in HSCT outcomes (i.e., progression-free survival, relapse/progression, and TRM), strictly connected to the transplant center experience, patient selection and progress with supportive care [8].
HSCT exerts its therapeutic effect in ADs through various mechanisms, including the immunosuppressive conditioning regimen able to eradicate the autoreactive immunologic memory, and the regeneration and renewal of the immune system, leading to the re-induction of immune tolerance to rewire aberrant immune response toward self-antigens [26, 34]. Moreover, changes in the microbiome profile [35], which have been linked to various ADs, merit further investigations in the context of immune recovery. The development of an immunological balance allows long-term disease remission [36].
Considerations for HSCT in neurological autoimmune diseases
Neurological ADs may affect the central and peripheral nervous systems, resulting in a variety of manifestations and symptoms. MS is currently the most frequent autoimmune disease for which HSCT has been used, accounting for 1875 patients reported in the EBMT registry (Fig. 1). Increasing evidence [5] support the use of autologous HSCT as highly effective therapeutic strategy for treatment-resistant inflammatory types of MS [33, 36,37,38,39,40], for which it can be currently regarded as a standard of care [6].
Remission of disease activity in patients with severe active MS undergoing autologous HSCT relies not only on the effect of high-dose chemotherapy and intense lymphodepletion, but also on the renewal [34] of the immune compartment. Initially conceived as an extreme “rescue” therapy in MS patients with a poor prognosis who have failed all other therapies, delivery of HSCT subsequently changed [41,42,43,44,45,46,47], including more patients in earlier inflammatory phases of the disease, with a gradual shift to a major predominance of relapsing remitting over progressive forms [8, 48]. This trend was also supported by the EBMT data [6].
Many patients, especially in Europe, have been conditioned with “intermediate intensity” conditioning regimens, such as BEAM (BCNU 300 mg/m2 on day −6, cytosine arabinoside, 200 mg/m2 and etoposide 200 mg/m2 day −5 to day −2, melphalan 140 mg/m2 day −1) plus rabbit ATG in dose range of 5–7.5 mg/kg, showing satisfactory toxicity/efficacy equipoise, even in the long-term follow-up [36]. Recently, low intensity regimens involving administration of Cy and ATG have been adopted, showing prolonged time to disease progression as compared to approved disease-modifying therapy (DMT) [33]. New studies are required to better define the optimal conditioning and solve this current regimen selection controversy. Importantly, autologous HSCT seems to offer clear advantage in terms of NEDA (no evidence of disease activity), showing rates of 66–93% compared with alemtuzumab, natalizumab or ocrelizumab [6].
In recent years, as clearly demonstrated by a marked decrease in TRM to 0.2%, better outcomes have been obtained owing to a growing experience in selecting the most appropriate patients to transplant, paralleled by advances in conditioning and support regimens [4, 8]. Despite the recent COVID-19 pandemic, the non-relapse mortality (NRM, defined as death for whatever cause, without ever experiencing relapse) remains stable around 1% from 2015 through all the 2020, according to the recent EBMT registry data.
Moreover, HSCT may be considered as “clinical option” in carefully selected patients affected by rare treatment-resistant neurological Ads [6]. Autologous HSCT is effective also in refractory and aggressive neuromyelitis optica [49, 50], chronic inflammatory demyelinating polyradiculoneuropathy [51], as well as myasthenia gravis [52], stiff-person syndrome disorder [53, 54], and anti-GAD (glutamic acid decarboxylase)-mediated encephalitis [55].
Considerations for HSCT in rheumatic diseases
Although the areas of application of HSCT for rheumatic diseases have changed over the past 25 years, they still represent major indications with 1158 patients reported to the EBMT registry to date (Fig. 1). Initially, HSCT has been applied for rheumatoid arthritis and juvenile idiopathic arthritis, where studies demonstrated mixed results with high rates of persistent or relapse of disease activity within 6 months of transplant requiring continued DMT [56, 57], not justifying the further development of HSCT in these indications. Subsequently, HSCT has been utilized for systemic lupus erythematosus (SLE) with more than 300 patients being treated worldwide so far [5]. Data from large single-center experiences and multicenter trials indicate a disease-free survival of ~50–66% at 5 years despite discontinuation of immunosuppressive and other targeted therapies [21, 58,59,60,61]. Responding patients are usually free of clinical symptoms and may regain seronegativity for antinuclear antibodies, which is rarely seen under conventional therapies. Early use of HSCT also has the potential to protect against organ-failure and toxicity-related morbidity and improve quality-of-life [61]. The relapse rate is higher in patients receiving unmanipulated stem cell grafts and conditioning regimens without serotherapy [60], potentially reflecting the reactivation of autoimmune responses initiated by residing long-lived memory plasma cells, which reasonably should be in the focus of future targeted approaches complementing Cy/ATG-based regimens. Current evidence and expert consensus suggest HSCT in SLE as “clinical option”, in patients with active disease despite chronic immunosuppression with or without B-cell-targeted therapies [62,63,64].
The main indication for HSCT has become rapidly progressive diffuse SSc, where even in the biologic’s era, effective therapies capable of reversing tissue fibrosis and improving lung function are lacking [5]. Three randomized-controlled trials (RCTs) comparing autologous HSCT to intravenous Cy in SSc reported significant improvement in skin and broader disease-specific scores, with evidence for improvement of pulmonary function, skin fibrosis and quality-of-life [65,66,67]. A 5-year progression-free survival between 70 and 74% was reported in the multicenter European (ASTIS) and American (SCOT) trials and remained superior to monthly Cy in the control arm during the 10 years following HSCT in the ASTIS trial. The increased toxicity of HSCT in SSc compared to other indications was predominantly attributed to SSc-related cardiac dysfunction, especially related to pulmonary arterial hypertension, and Cy-induced cardiotoxicity. With improved screening procedures [68, 69], as well as Cy-sparing conditioning regimens, TRM decreased from 10% in the ASTIS trial to 6% within a multicenter EBMT prospective study [7], 3% in the SCOT trial [67], and 2.4% in the CAST study [70]. Based on these data, SSc is recommended as ‘standard indication’ for autologous HSCT and endorsed by updated recommendations of the European League Against Rheumatism [71]. Rarer indications of HSCT for rheumatic diseases with positive results from retrospective EBMT studies include ANCA-associated vasculitis [72], Takayasu arteritis [73] and Behcet’s disease [74].
Considerations for HSCT in gastrointestinal diseases
The main area application of HSCT for gastroenterologic disease has been in inflammatory bowel disease [5], particularly Crohn’s Disease (CD) with 215 patients reported to the EBMT registry to date (Fig. 1). In addition to single-arm series [75,76,77,78,79], including a large EBMT retrospective study [80], one RCT, the EBMT-sponsored ASTIC trial, has reported promising results. Although the ambiguous endpoint of the ASTIC study, i.e., no evidence of active disease on endoscopy or imaging with a CD activity index <150 for at least 3 months while off all CD medications, was not achieved, data supported improvement in clinical and endoscopic measures of activity and quality-of-life, with 50% of patients showing complete endoscopic mucosal healing [81]. Accordingly, CD is suggested as “clinical option” for HSCT and the EBMT and the European Crohn’s and Colitis Organization (ECCO) provided a collaborative position paper to guide the current practice and forward development of HSCT in CD [82]. Future developments in the field include the utilization of low intensity regimens, currently investigated in the ASTIC-lite study [83], and the use of umbilical cord blood (UCB) allogeneic HSCT, which already demonstrated a 5-year long clinical, endoscopic, and histologic disease-free and drug-free survival that occurred unexpectedly without donor engraftment or risk of graft-versus-host disease (GvHD) [84].
Considerations for allogeneic HSCT
Allogeneic HSCT is widely used to treat patients with malignant and nonmalignant hematological disorders [1]. This strategy may represent an attractive option for patients with refractory ADs, theoretically offering the advantage of complete eradication of autoreactive cells combined with the regeneration of a healthy immune system, tolerant to autoantigens [85]. In this context, the donor immune system plays a pivotal role in promoting a putative donor-versus-host alloreactivity [86, 87].
During the last decade major changes have occurred in the field of allogeneic HSCT [88,89,90], including the introduction of less aggressive conditioning regimens [91, 92], improved patient selection, and better supportive care, with a substantial progress in reducing GvHD because of more accurate HLA-typing and better GvHD prevention drugs, opening this procedure also to nonmalignant disorders [93,94,95,96]. Progressively the choice of donors and the sources of HSCs have enlarged, extending transplant indications to more patients [97]. Despite improved survival over time [98], allogeneic HSCT use in AD has remained rare and largely restricted to immune cytopenia, pediatric practice, and carefully selected patients affected by monogenic autoinflammatory disorders presenting with “rheumatic” phenotype [96, 99,100,101]. A retrospective EBMT study reported better long-term outcomes in younger patients with more recent year of transplant [85]. However, its potential to provide long-term disease control in refractory ADs paves the way for extended clinical trials to better investigate its role mainly in younger patients [85].
Innovative cellular strategies in ADs
Alongside HSCT, innovative therapy approaches with immune-regulatory capacities such as mesenchymal stromal cells (MSC) and regulatory T cells (Tregs) have been developed over the last decades for treating severe Ads [5]. Published trials on advanced cellular therapies in this population are safe, while showing controversial beneficial effects, mainly due to a poor specificity of cell products related to a low number of true disease-relevant antigen-specific cells [102,103,104,105]. The great variability among different trials and small numbers of patients warrants further studies in this setting.
Additionally, chimeric antigen receptor (CAR)-T cells [106], one of the most promising therapy approaches for hematological malignancies, may be employed in the field of autoimmunity [107], thanks to their ability of conferring new antigen-specificities and contemporary boosting cell activation. Early data on the successful treatment of a patient with refractory SLE with CD19-targeted CAR-T cells has been recently reported [108], showing encouraging results and paving the way to this new cell strategy.
Moreover, stem cells represent an important source for potential therapeutic interventions in regenerative medicine [5]. Among them, induced pluripotent stem cells (IPS) probably resemble the most promising future approach. IPS are generated from somatic cells that have been reprogrammed by the ectopic expression of defined embryonic transcription factors (typically: Oct3/4, Sox2, c-Myc, Klf4) called “Yamanaka factors” (OSKM) [109]. They are currently under investigation for the treatment of Parkinson’s disease, macular degeneration and type I diabetes mellitus. In addition, neuronal stem cells (NSC) are increasingly evaluated as potential source for replacing damaged or lost neurons and glia [110]. Their therapeutic application already demonstrated promising results in the context of spinal cord injury in rodents [111]. Other areas of investigation include the exploitation of the inherent biologic advantages of UCB stem cells with high proliferative potential and good damage repair capacity [112], and the biologic properties, therapeutic mechanisms and clinical efficacy of MSC or MSC-derived exosomes [113, 114].
International registries: activity of HSCT in autoimmune diseases
Two major transplant registries, the European Society for Blood and Marrow Transplantation (EBMT) and the Center for International Blood and Marrow Transplant Research (CIBMTR), capture data from many ADs patients [5].
In EBMT, quality in HSCT and cellular therapy is assured through the Joint Accreditation Committee of the International Society for Cellular Therapy and EBMT (JACIE) accreditation, which has been central to EBMT recommendations and may potentially affect the outcome of HSCT [8]. The EBMT Autoimmune Diseases Working Party (ADWP) is central to bringing together HSCT and disease specialist communities. The ADs section of the EBMT Registry is the largest database of its kind worldwide (Figs. 1, 2). As of February 2022, a total of 3789 HSCT procedures (autologous in 93% of cases) for autoimmune indications have been reported in the EBMT registry. The CIBMTR Research Database captures outcomes of patients who receive an HSCT and follows patients longitudinally until death or lost to follow-up. The CIBMTR’s study of HSCT for AID is overseen by the Nonmalignant Disease Working Committee. In the future, registry activity will continue to be essential for the further development of the field.
Conclusions
Current evidence support HSCT as a valid treatment option in the management of selected patients with ADs, with a clear risk/benefit ratio as carefully evaluated by appropriately constituted multidisciplinary team (including transplant and disease specialists). HSCT can induce lasting remissions in patients with severe autoimmune disorders, underlying the value of these therapeutic approaches and the potential for immune reconstitution and immune resetting of T and B-cell repertoire. Center experience, accreditation, inter-specialty networking, and national socioeconomic factors are relevant for health service delivery of HSCT in ADs. New insights are coming also in the complexity and power of innovative cellular therapies. Future studies are warranted to further elucidate the mechanism of action of these cellular-based therapies, while shedding light on the underlying pathogenesis of ADs.
Data availability
The datasets generated for this study are available on request to the corresponding author.
References
Passweg JR, Baldomero H, Chabannon C, Basak GW, de la Camara R, Corbacioglu S, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021;56:1651–64. https://doi.org/10.1038/s41409-021-01227-8.
Alexander T, Greco R, Snowden JA. Hematopoietic stem cell transplantation for autoimmune disease. Annu Rev Med. 2021;72:215–28. https://doi.org/10.1146/annurev-med-070119-115617.
Greco R, Alexander T, Burman J, Del Papa N, de Vries-Bouwstra J, Farge D, et al. Hematopoietic stem cell transplantation for autoimmune diseases in the time of COVID-19: EBMT guidelines and recommendations. Bone Marrow Transplant. 2021;56:1493–508. https://doi.org/10.1038/s41409-021-01326-6.
Muraro PA, Martin R, Mancardi GL, Nicholas R, Sormani MP, Saccardi R. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat Rev Neurol. 2017;13:391–405. https://doi.org/10.1038/nrneurol.2017.81.
Burt RK, Farge D, Ruiz MA, Saccardi R, Snowden JA. Hematopoietic stem cell transplantation and cellular therapies for autoimmune diseases. 2021. eBook: 978-1-315-15136-6: https://www.routledge.com/Hematopoietic-Stem-Cell-Transplantation-and-Cellular-Therapies-for-Autoimmune/Burt-Farge-Ruiz-Saccardi-Snowden/p/book/978113855855.
Sharrack B, Saccardi R, Alexander T, Badoglio M, Burman J, Farge D, et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transplant. 2020;55:283–306. https://doi.org/10.1038/s41409-019-0684-0.
Henes J, Oliveira MC, Labopin M, Badoglio M, Scherer HU, Del Papa N, et al. Autologous stem cell transplantation for progressive systemic sclerosis: a prospective non-interventional study from the European Society for Blood and Marrow Transplantation Autoimmune Disease Working Party. Haematologica. 2021;106:375–83. https://doi.org/10.3324/haematol.2019.230128.
Snowden JA, Badoglio M, Labopin M, Giebel S, McGrath E, Marjanovic Z, et al. Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood Adv. 2017;1:2742–55. https://doi.org/10.1182/bloodadvances.2017010041.
Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000;908:244–54. https://doi.org/10.1111/j.1749-6632.2000.tb06651.x.
McKenna RW, Washington LT, Aquino DB, Picker LJ, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) in 662 consecutive bone marrow specimens by 4-color flow cytometry. Blood. 2001;98:2498–507. https://doi.org/10.1182/blood.v98.8.2498.
Frasca D, Diaz A, Romero M, D’Eramo F, Blomberg BB. Aging effects on T-bet expression in human B cell subsets. Cell Immunol. 2017;321:68–73. https://doi.org/10.1016/j.cellimm.2017.04.007.
Verma N, Dimitrova M, Carter DM, Crevar CJ, Ross TM, Golding H, et al. Influenza virus H1N1pdm09 infections in the young and old: evidence of greater antibody diversity and affinity for the hemagglutinin globular head domain (HA1 Domain) in the elderly than in young adults and children. J Virol. 2012;86:5515–22. https://doi.org/10.1128/JVI.07085-11.
Goronzy JJ, Weyand CM. Successful and maladaptive T cell aging. Immunity. 2017;46:364–78. https://doi.org/10.1016/j.immuni.2017.03.010.
Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, Lee JY, et al. Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci USA. 2014;111:13139–44. https://doi.org/10.1073/pnas.1409155111.
Pera A, Campos C, Lopez N, Hassouneh F, Alonso C, Tarazona R, et al. Immunosenescence: Implications for response to infection and vaccination in older people. Maturitas. 2015;82:50–5. https://doi.org/10.1016/j.maturitas.2015.05.004.
Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–4. https://doi.org/10.1038/40540.
Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–72.
Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–50. https://doi.org/10.1038/nri1886.
Hiepe F, Dorner T, Hauser AE, Hoyer BF, Mei H, Radbruch A. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol. 2011;7:170–8. https://doi.org/10.1038/nrrheum.2011.1.
Hiepe F, Radbruch A. Plasma cells as an innovative target in autoimmune disease with renal manifestations. Nat Rev Nephrol. 2016;12:232–40. https://doi.org/10.1038/nrneph.2016.20.
Alexander T, Thiel A, Rosen O, Massenkeil G, Sattler A, Kohler S, et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood. 2009;113:214–23. https://doi.org/10.1182/blood-2008-07-168286.
Arruda LCM, Malmegrim KCR, Lima-Junior JR, Clave E, Dias JBE, Moraes DA, et al. Immune rebound associates with a favorable clinical response to autologous HSCT in systemic sclerosis patients. Blood Adv. 2018;2:126–41. https://doi.org/10.1182/bloodadvances.2017011072.
Muraro PA, Douek DC, Packer A, Chung K, Guenaga FJ, Cassiani-Ingoni R, et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med. 2005;201:805–16. https://doi.org/10.1084/jem.20041679.
Farge D, Arruda LC, Brigant F, Clave E, Douay C, Marjanovic Z, et al. Long-term immune reconstitution and T cell repertoire analysis after autologous hematopoietic stem cell transplantation in systemic sclerosis patients. J Hematol Oncol. 2017;10:21. https://doi.org/10.1186/s13045-016-0388-5.
Alexander T, Sattler A, Templin L, Kohler S, Gross C, Meisel A, et al. Foxp3+ Helios+ regulatory T cells are expanded in active systemic lupus erythematosus. Ann Rheum Dis. 2013;72:1549–58. https://doi.org/10.1136/annrheumdis-2012-202216.
Lima-Junior JR, Arruda LCM, Goncalves MS, Dias JBE, Moraes DA, Covas DT, et al. Autologous haematopoietic stem cell transplantation restores the suppressive capacity of regulatory B cells in systemic sclerosis patients. Rheumatology. 2021;60:5538–48. https://doi.org/10.1093/rheumatology/keab257.
Muraro PA, Robins H, Malhotra S, Howell M, Phippard D, Desmarais C, et al. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J Clin Investig. 2014;124:1168–72. https://doi.org/10.1172/JCI71691.
Harris KM, Lim N, Lindau P, Robins H, Griffith LM, Nash RA, et al. Extensive intrathecal T cell renewal following hematopoietic transplantation for multiple sclerosis. JCI Insight. 2020;5. https://doi.org/10.1172/jci.insight.127655.
Kelsey PJ, Oliveira MC, Badoglio M, Sharrack B, Farge D, Snowden JA. Haematopoietic stem cell transplantation in autoimmune diseases: from basic science to clinical practice. Curr Res Transl Med. 2016;64:71–82. https://doi.org/10.1016/j.retram.2016.03.003.
Alexander T, Farge D, Badoglio M, Lindsay JO, Muraro PA, Snowden JA, et al. Hematopoietic stem cell therapy for autoimmune diseases—clinical experience and mechanisms. J Autoimmun. 2018;92:35–46. https://doi.org/10.1016/j.jaut.2018.06.002.
Oliveira MC, Labopin M, Henes J, Moore J, Del Papa N, Cras A, et al. Does ex vivo CD34+ positive selection influence outcome after autologous hematopoietic stem cell transplantation in systemic sclerosis patients? Bone Marrow Transplant. 2016;51:501–5. https://doi.org/10.1038/bmt.2015.299.
Gagelmann N, Kroger N. Dose intensity for conditioning in allogeneic hematopoietic cell transplantation: can we recommend “when and for whom” in 2021? Haematologica. 2021;106:1794–804. https://doi.org/10.3324/haematol.2020.268839.
Burt RK, Balabanov R, Burman J, Sharrack B, Snowden JA, Oliveira MC, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA. 2019;321:165–74. https://doi.org/10.1001/jama.2018.18743.
Cencioni MT, Genchi A, Brittain G, de Silva T, Sharrack B, Snowden JA, et al. Immune reconstitution following autologous hematopoietic stem cell transplantation for multiple sclerosis: a review on behalf of the EBMT Autoimmune Diseases Working Party (ADWP). Front Immunol. 2022;12:813957. https://doi.org/10.3389/fimmu.2021.813957.
Alexander T, Snowden JA, Burman J, Chang HD, Del Papa N, Farge D, et al. Intestinal microbiome in hematopoietic stem cell transplantation for autoimmune diseases: considerations and perspectives on behalf of autoimmune diseases working party (ADWP) of the EBMT. Front Oncol. 2021;11:722436. https://doi.org/10.3389/fonc.2021.722436.
Muraro PA, Pasquini M, Atkins HL, Bowen JD, Farge D, Fassas A, et al. Long-term Outcomes After Autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol. 2017;74:459–69. https://doi.org/10.1001/jamaneurol.2016.5867.
Boffa G, Massacesi L, Inglese M, Mariottini A, Capobianco M, Lucia M, et al. Long-term clinical outcomes of hematopoietic stem cell transplantation in multiple sclerosis. Neurology. 2021. https://doi.org/10.1212/WNL.0000000000011461.
Das J, Snowden JA, Burman J, Freedman MS, Atkins H, Bowman M, et al. Autologous haematopoietic stem cell transplantation as a first-line disease-modifying therapy in patients with ‘aggressive’ multiple sclerosis. Mult Scler. 2021;27:1198–204. https://doi.org/10.1177/1352458520985238.
Tappenden P, Wang Y, Sharrack B, Burman J, Kazmi M, Saccardi R, et al. Evaluating the clinical effectiveness of autologous haematopoietic stem cell transplantation versus disease-modifying therapy in multiple sclerosis using a matching-adjusted indirect comparison: an exploratory study from the Autoimmune Diseases Working Party (ADWP) of the European Society of Bone and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2020;55:1473–5. https://doi.org/10.1038/s41409-019-0747-2.
Mancardi GL, Sormani MP, Gualandi F, Saiz A, Carreras E, Merelli E, et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a phase II trial. Neurology. 2015;84:981–8. https://doi.org/10.1212/WNL.0000000000001329.
Mancardi GL, Sormani MP, Di Gioia M, Vuolo L, Gualandi F, Amato MP, et al. Autologous haematopoietic stem cell transplantation with an intermediate intensity conditioning regimen in multiple sclerosis: the Italian multi-centre experience. Mult Scler. 2012;18:835–42. https://doi.org/10.1177/1352458511429320.
Burman J, Iacobaeus E, Svenningsson A, Lycke J, Gunnarsson M, Nilsson P, et al. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: the Swedish experience. J Neurol Neurosurg Psychiatry. 2014;85:1116–21. https://doi.org/10.1136/jnnp-2013-307207.
Shevchenko JL, Kuznetsov AN, Ionova TI, Melnichenko VY, Fedorenko DA, Kartashov AV, et al. Autologous hematopoietic stem cell transplantation with reduced-intensity conditioning in multiple sclerosis. Exp Hematol. 2012;40:892–8. https://doi.org/10.1016/j.exphem.2012.07.003.
Nash RA, Hutton GJ, Racke MK, Popat U, Devine SM, Griffith LM, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for relapsing-remitting multiple sclerosis (HALT-MS): a 3-year interim report. JAMA Neurol. 2015;72:159–69. https://doi.org/10.1001/jamaneurol.2014.3780.
Burt RK, Balabanov R, Han X, Sharrack B, Morgan A, Quigley K, et al. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA. 2015;313:275–84. https://doi.org/10.1001/jama.2014.17986.
Radaelli M, Merlini A, Greco R, Sangalli F, Comi G, Ciceri F, et al. Autologous bone marrow transplantation for the treatment of multiple sclerosis. Curr Neurol Neurosci Rep. 2014;14:478. https://doi.org/10.1007/s11910-014-0478-0.
Burt RK, Balabanov R, Voltarelli J, Barreira A, Burman J. Autologous hematopoietic stem cell transplantation for multiple sclerosis-if confused or hesitant, remember: ‘treat with standard immune suppressive drugs and if no inflammation, no response’. Mult Scler. 2012;18:772–5. https://doi.org/10.1177/1352458512442993.
Saccardi R, Freedman MS, Sormani MP, Atkins H, Farge D, Griffith LM, et al. A prospective, randomized, controlled trial of autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: a position paper. Mult Scler. 2012;18:825–34. https://doi.org/10.1177/1352458512438454.
Greco R, Bondanza A, Oliveira MC, Badoglio M, Burman J, Piehl F, et al. Autologous hematopoietic stem cell transplantation in neuromyelitis optica: a registry study of the EBMT Autoimmune Diseases Working Party. Mult Scler. 2015;21:189–97. https://doi.org/10.1177/1352458514541978.
Burt RK, Balabanov R, Han X, Burns C, Gastala J, Jovanovic B, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation for neuromyelitis optica. Neurology. 2019;93:e1732–41. https://doi.org/10.1212/WNL.0000000000008394.
Burt RK, Balabanov R, Tavee J, Han X, Sufit R, Ajroud-Driss S, et al. Hematopoietic stem cell transplantation for chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol. 2020;267:3378–91. https://doi.org/10.1007/s00415-020-10010-6.
Bryant A, Atkins H, Pringle CE, Allan D, Anstee G, Bence-Bruckler I, et al. Myasthenia gravis treated with autologous hematopoietic stem cell transplantation. JAMA Neurol. 2016;73:652–8. https://doi.org/10.1001/jamaneurol.2016.0113.
Kass-Iliyya L, Snowden JA, Thorpe A, Jessop H, Chantry AD, Sarrigiannis PG, et al. Autologous haematopoietic stem cell transplantation for refractory stiff-person syndrome: the UK experience. J Neurol. 2021;268:265–75. https://doi.org/10.1007/s00415-020-10054-8.
Burt RK, Balabanov R, Han X, Quigley K, Arnautovic I, Helenowski I, et al. Autologous Hematopoietic Stem Cell Transplantation for Stiff-Person Spectrum Disorder: A Clinical Trial. Neurology. 2021;96:e817–30. https://doi.org/10.1212/WNL.0000000000011338.
Abrahamsson SV, Angelini DF, Dubinsky AN, Morel E, Oh U, Jones JL, et al. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain. 2013;136:2888–903. https://doi.org/10.1093/brain/awt182.
Snowden JA, Passweg J, Moore JJ, Milliken S, Cannell P, Van Laar J, et al. Autologous hemopoietic stem cell transplantation in severe rheumatoid arthritis: a report from the EBMT and ABMTR. J Rheumatol. 2004;31:482–8.
De Kleer IM, Brinkman DM, Ferster A, Abinun M, Quartier P, Van Der Net J, et al. Autologous stem cell transplantation for refractory juvenile idiopathic arthritis: analysis of clinical effects, mortality, and transplant related morbidity. Ann Rheum Dis. 2004;63:1318–26. https://doi.org/10.1136/ard.2003.017798.
Jayne D, Passweg J, Marmont A, Farge D, Zhao X, Arnold R, et al. Autologous stem cell transplantation for systemic lupus erythematosus. Lupus. 2004;13:168–76.
Burt RK, Traynor A, Statkute L, Barr WG, Rosa R, Schroeder J, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA. 2006;295:527–35. https://doi.org/10.1001/jama.295.5.527.
Alchi B, Jayne D, Labopin M, Demin A, Sergeevicheva V, Alexander T, et al. Autologous haematopoietic stem cell transplantation for systemic lupus erythematosus: data from the European Group for Blood and Marrow Transplantation registry. Lupus. 2013;22:245–53. https://doi.org/10.1177/0961203312470729.
Burt RK, Han X, Gozdziak P, Yaung K, Morgan A, Clendenan AM, et al. Five year follow-up after autologous peripheral blood hematopoietic stem cell transplantation for refractory, chronic, corticosteroid-dependent systemic lupus erythematosus: effect of conditioning regimen on outcome. Bone Marrow Transplant. 2018;53:692–700. https://doi.org/10.1038/s41409-018-0173-x.
Duarte RF, Labopin M, Bader P, Basak GW, Bonini C, Chabannon C, et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant. 2019. https://doi.org/10.1038/s41409-019-0516-2.
Illei GG, Cervera R, Burt RK, Doria A, Hiepe F, Jayne D, et al. Current state and future directions of autologous hematopoietic stem cell transplantation in systemic lupus erythematosus. Ann Rheum Dis. 2011;70:2071–4. https://doi.org/10.1136/ard.2010.148049.
Snowden JA, Saccardi R, Allez M, Ardizzone S, Arnold R, Cervera R, et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012;47:770–90. https://doi.org/10.1038/bmt.2011.185.
Burt RK, Shah SJ, Dill K, Grant T, Gheorghiade M, Schroeder J, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet. 2011;378:498–506. https://doi.org/10.1016/S0140-6736(11)60982-3.
van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA. 2014;311:2490–8. https://doi.org/10.1001/jama.2014.6368.
Sullivan KM, Goldmuntz EA, Furst DE. Autologous Stem-Cell Transplantation for Severe Scleroderma. N. Engl J Med. 2018;378:1066–7. https://doi.org/10.1056/NEJMc1801275.
Burt RK, Oliveira MC, Shah SJ. Cardiac assessment before stem cell transplantation for systemic sclerosis. JAMA. 2014;312:1803. https://doi.org/10.1001/jama.2014.12566.
Farge D, Burt RK, Oliveira MC, Mousseaux E, Rovira M, Marjanovic Z, et al. Cardiopulmonary assessment of patients with systemic sclerosis for hematopoietic stem cell transplantation: recommendations from the European Society for Blood and Marrow Transplantation Autoimmune Diseases Working Party and collaborating partners. Bone Marrow Transplant. 2017;52:1495–503. https://doi.org/10.1038/bmt.2017.56.
Burt RK, Han X, Quigley K, Arnautovic I, Shah SJ, Lee DC, et al. Cardiac safe hematopoietic stem cell transplantation for systemic sclerosis with poor cardiac function: a pilot safety study that decreases neutropenic interval to 5 days. Bone Marrow Transplant. 2021;56:50–9. https://doi.org/10.1038/s41409-020-0978-2.
Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76:1327–39. https://doi.org/10.1136/annrheumdis-2016-209909.
Alexander T, Samuelson C, Daikeler T, Henes J, Akil M, Skagerlind L, et al. Autologous haematopoietic stem cell transplantation (HSCT) for anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis: a retrospective survey of patients reported to European Society for Blood and Marrow Transplantation (EBMT) registry. Bone Marrow Transplant. 2020;55:1512–5. https://doi.org/10.1038/s41409-019-0763-2.
Laurent C, Marjanovic Z, Ricard L, Henes J, Dulery R, Badoglio M, et al. Autologous hematopoietic stem cell transplantation with reduced-intensity conditioning regimens in refractory Takayasu arteritis: a retrospective multicenter case-series from the Autoimmune Diseases Working Party (ADWP) of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2020;55:2109–13. https://doi.org/10.1038/s41409-020-0907-4.
Puyade M, Patel A, Lim YJ, Blank N, Badoglio M, Gualandi F, et al. Autologous Hematopoietic Stem Cell Transplantation for Behcet’s Disease: A Retrospective Survey of Patients Treated in Europe, on Behalf of the Autoimmune Diseases Working Party of the European Society for Blood and Marrow Transplantation. Front Immunol. 2021;12:638709. https://doi.org/10.3389/fimmu.2021.638709.
Oyama Y, Craig RM, Traynor AE, Quigley K, Statkute L, Halverson A, et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn’s disease. Gastroenterology. 2005;128:552–63. https://doi.org/10.1053/j.gastro.2004.11.051.
Snowden JA, Ansari A, Sachchithanantham S, Jackson G, Thompson N, Lobo A, et al. Autologous stem cell transplantation in severe treatment-resistant Crohn’s disease: long-term follow-up of UK patients treated on compassionate basis. QJM. 2014;107:871–7. https://doi.org/10.1093/qjmed/hcu095.
Jauregui-Amezaga A, Rovira M, Marin P, Salas A, Pino-Donnay S, Feu F, et al. Improving safety of autologous haematopoietic stem cell transplantation in patients with Crohn’s disease. Gut. 2016;65:1456–62. https://doi.org/10.1136/gutjnl-2015-309836.
Lopez-Garcia A, Rovira M, Jauregui-Amezaga A, Marin P, Barastegui R, Salas A, et al. Autologous haematopoietic stem cell transplantation for refractory Crohn’s disease: efficacy in a single-centre cohort. J Crohns Colitis. 2017;11:1161–8. https://doi.org/10.1093/ecco-jcc/jjx054.
Ruiz MA, Kaiser RL Jr., de Quadros LG, Piron-Ruiz L, Pena-Arciniegas T, Faria MAG, et al. Low toxicity and favorable clinical and quality of life impact after non-myeloablative autologous hematopoietic stem cell transplant in Crohn’s disease. BMC Res Notes. 2017;10:495. https://doi.org/10.1186/s13104-017-2824-1.
Brierley CK, Castilla-Llorente C, Labopin M, Badoglio M, Rovira M, Ricart E, et al. Autologous haematopoietic stem cell transplantation for crohn’s disease: a retrospective survey of long-term outcomes from the European Society for Blood and Marrow Transplantation. J Crohns Colitis. 2018;12:1097–103. https://doi.org/10.1093/ecco-jcc/jjy069.
Lindsay JO, Allez M, Clark M, Labopin M, Ricart E, Rogler G, et al. Autologous stem-cell transplantation in treatment-refractory Crohn’s disease: an analysis of pooled data from the ASTIC trial. Lancet Gastroenterol Hepatol. 2017;2:399–406. https://doi.org/10.1016/S2468-1253(17)30056-0.
Snowden JA, Panes J, Alexander T, Allez M, Ardizzone S, Dierickx D, et al. Autologous haematopoietic stem cell transplantation (AHSCT) in severe Crohn’s disease: a review on behalf of ECCO and EBMT. J Crohns Colitis. 2018;12:476–88. https://doi.org/10.1093/ecco-jcc/jjx184.
Snowden JA, Hawkey C, Hind D, Swaby L, Mellor K, Emsley R, et al. Autologous stem cell transplantation in refractory Crohn’s disease - low intensity therapy evaluation (ASTIClite): study protocols for a multicentre, randomised controlled trial and observational follow up study. BMC Gastroenterol. 2019;19:82. https://doi.org/10.1186/s12876-019-0992-2.
Burt RK, Craig R, Yun L, Halverson A, Quigley K, Arnautovic I, et al. A pilot feasibility study of non-myeloablative allogeneic hematopoietic stem cell transplantation for refractory Crohn Disease. Bone Marrow Transplant. 2020;55:2343–6. https://doi.org/10.1038/s41409-020-0953-y.
Greco R, Labopin M, Badoglio M, Veys P, Furtado Silva JM, Abinun M, et al. Allogeneic HSCT for autoimmune diseases: a retrospective study from the EBMT ADWP, IEWP, and PDWP working parties. Front Immunol. 2019;10:1570. https://doi.org/10.3389/fimmu.2019.01570.
Herrmann MM, Gaertner S, Stadelmann C, van den Brandt J, Boscke R, Budach W, et al. Tolerance induction by bone marrow transplantation in a multiple sclerosis model. Blood. 2005;106:1875–83. https://doi.org/10.1182/blood-2004-12-4607.
Van Wijmeersch B, Sprangers B, Rutgeerts O, Lenaerts C, Landuyt W, Waer M, et al. Allogeneic bone marrow transplantation in models of experimental autoimmune encephalomyelitis: evidence for a graft-versus-autoimmunity effect. Biol Blood Marrow Transplant. 2007;13:627–37. https://doi.org/10.1016/j.bbmt.2007.03.001.
Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res. 2010;47:65–77. https://doi.org/10.1007/s12026-009-8139-0.
Bonifazi F, Rubio MT, Bacigalupo A, Boelens JJ, Finke J, Greinix H, et al. Rabbit ATG/ATLG in preventing graft-versus-host disease after allogeneic stem cell transplantation: consensus-based recommendations by an international expert panel. Bone Marrow Transplant. 2020. https://doi.org/10.1038/s41409-020-0792-x.
Greco R, Lorentino F, Albanese S, Teresa Lupo Stanghellini M, Giglio F, Piemontese S, et al. Post-transplant cyclophosphamide and sirolimus based graft-versus-host-disease prophylaxis in allogeneic stem cell transplant. Transplant Cell Ther. 2021. https://doi.org/10.1016/j.jtct.2021.05.023.
Beelen DW, Trenschel R, Stelljes M, Groth C, Masszi T, Remenyi P, et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT.14/L): a randomised, non-inferiority, phase 3 trial. Lancet Haematol. 2020;7:e28–39. https://doi.org/10.1016/S2352-3026(19)30157-7.
Lazzari L, Ruggeri A, Lupo Stanghellini MT, Mastaglio S, Messina C, Giglio F, et al. Treosulfan-based conditioning regimen prior to allogeneic stem cell transplantation: long-term results from a Phase 2 clinical trial. Front Oncol. 2021;11:731478. https://doi.org/10.3389/fonc.2021.731478.
Greco R, Bondanza A, Vago L, Moiola L, Rossi P, Furlan R, et al. Allogeneic hematopoietic stem cell transplantation for neuromyelitis optica. Ann Neurol. 2014;75:447–53. https://doi.org/10.1002/ana.24079.
Ceglie G, Papetti L, Figa Talamanca L, Lucarelli B, Algeri M, Gaspari S, et al. T-cell depleted HLA-haploidentical HSCT in a child with neuromyelitis optica. Ann Clin Transl Neurol. 2019;6:2110–3. https://doi.org/10.1002/acn3.50843.
J MFS, Ladomenou F, Carpenter B, Chandra S, Sedlacek P, Formankova R, et al. Allogeneic hematopoietic stem cell transplantation for severe, refractory juvenile idiopathic arthritis. Blood Adv. 2018;2:777–86. https://doi.org/10.1182/bloodadvances.2017014449.
Abinun M, Slatter MA. Haematopoietic stem cell transplantation in paediatric rheumatic disease. Curr Opin Rheumatol. 2021;33:387–97. https://doi.org/10.1097/BOR.0000000000000823.
Ringden O, Boumendil A, Labopin M, Canaani J, Beelen D, Ehninger G, et al. Outcome of Allogeneic Hematopoietic Stem Cell Transplantation in Patients Age >69 Years with Acute Myelogenous Leukemia: On Behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2019;25:1975–83. https://doi.org/10.1016/j.bbmt.2019.05.037.
Penack O, Peczynski C, Mohty M, Yakoub-Agha I, Styczynski J, Montoto S, et al. How much has allogeneic stem cell transplant-related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv. 2020;4:6283–90. https://doi.org/10.1182/bloodadvances.2020003418.
Daikeler T, Hugle T, Farge D, Andolina M, Gualandi F, Baldomero H, et al. Allogeneic hematopoietic SCT for patients with autoimmune diseases. Bone Marrow Transplant. 2009;44:27–33. https://doi.org/10.1038/bmt.2008.424.
Rabusin M, Snowden JA, Veys P, Quartier P, Dalle JH, Dhooge C, et al. Long-term outcomes of hematopoietic stem cell transplantation for severe treatment-resistant autoimmune cytopenia in children. Biol Blood Marrow Transplant. 2013;19:666–9. https://doi.org/10.1016/j.bbmt.2012.12.008.
Oyama Y, Traynor AE, Barr W, Burt RK. Allogeneic stem cell transplantation for autoimmune diseases: nonmyeloablative conditioning regimens. Bone Marrow Transplant. 2003;32 Suppl 1:S81–3. https://doi.org/10.1038/sj.bmt.1703950.
Wang D, Zhang H, Liang J, Li X, Feng X, Wang H, et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013;22:2267–77. https://doi.org/10.3727/096368911X582769c.
Velier M, Daumas A, Simoncini S, Arcani R, Magalon J, Benyamine A, et al. Combining systemic and locally applied cellular therapies for the treatment of systemic sclerosis. Bone Marrow Transplant. 2022;57:17–22. https://doi.org/10.1038/s41409-021-01492-7.
Uccelli A, Laroni A, Ali R, Battaglia MA, Blinkenberg M, Brundin L, et al. Safety, tolerability, and activity of mesenchymal stem cells versus placebo in multiple sclerosis (MESEMS): a phase 2, randomised, double-blind crossover trial. Lancet Neurol. 2021;20:917–29. https://doi.org/10.1016/S1474-4422(21)00301-X.
Romano M, Fanelli G, Albany CJ, Giganti G, Lombardi G. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front Immunol. 2019;10:43. https://doi.org/10.3389/fimmu.2019.00043.
Hayden PJ, Roddie C, Bader P, Basak GW, Bonig H, Bonini C, et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann Oncol. 2021. https://doi.org/10.1016/j.annonc.2021.12.003.
Sadeqi Nezhad M, Seifalian A, Bagheri N, Yaghoubi S, Karimi MH, Adbollahpour-Alitappeh M. Chimeric antigen receptor based therapy as a potential approach in autoimmune diseases: how close are we to the treatment? Front Immunol. 2020;11:603237. https://doi.org/10.3389/fimmu.2020.603237.
Mougiakakos D, Kronke G, Volkl S, Kretschmann S, Aigner M, Kharboutli S, et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N Engl J Med. 2021;385:567–9. https://doi.org/10.1056/NEJMc2107725.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. https://doi.org/10.1016/j.cell.2006.07.024.
Ottoboni L, von Wunster B, Martino G. Therapeutic plasticity of neural stem cells. Front Neurol. 2020;11:148. https://doi.org/10.3389/fneur.2020.00148.
Hong JY, Lee SH, Lee SC, Kim JW, Kim KP, Kim SM, et al. Therapeutic potential of induced neural stem cells for spinal cord injury. J Biol Chem. 2014;289:32512–25. https://doi.org/10.1074/jbc.M114.588871.
Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–35. https://doi.org/10.1084/jem.20040440.
Shen Z, Huang W, Liu J, Tian J, Wang S, Rui K. Effects of mesenchymal stem cell-derived exosomes on autoimmune diseases. Front Immunol. 2021;12:749192. https://doi.org/10.3389/fimmu.2021.749192.
Kouchakian MR, Baghban N, Moniri SF, Baghban M, Bakhshalizadeh S, Najafzadeh V, et al. The clinical trials of mesenchymal stromal cells therapy. Stem Cells Int. 2021;2021:1634782. https://doi.org/10.1155/2021/1634782.
Acknowledgements
The authors contribute this article on behalf of ADWP of the EBMT. The authors thank Manuela Badoglio and Myriam Labopin in the EBMT Paris Office for provision of data from the EBMT registry, EBMT centers for their contributions to the registry and those active in the ADWP. The authors thank Prof. Burt, Prof. Farge, Prof. Ruiz, Prof. Saccardi, and Prof. Snowden for the effort in creating a huge international reference work in the context of ADs. The authors are profoundly grateful to Prof. Burt also for his clinical and research practice of HSCT in ADs, a field in which he has been one of the world leaders, and, indisputable, the principal pioneer in the field based upon his major contributions.
Author information
Authors and Affiliations
Contributions
RG and TA led on concept, design, coordination and data review, provided expert and analytical feedback and were both involved in reviewing, writing and editing the paper. The two co-authors were involved in drafting the paper, revising it critically, and approval of the submitted and final versions.
Corresponding authors
Ethics declarations
Competing interests
RG discloses speaking honoraria from Biotest, Pfizer, Medac, and Magenta. TA declares speaking honoraria from Amgen, Roche, Pfizer, travel grants from Neovii, and study support from Amgen, Janssen-Cilag, and Miltenyi Biotec.
Ethics approval
This review was led and supported by the EBMT-ADWP. The EBMT provided resources via the working party, data office, and registry. Other than EBMT support there is no funding body supporting these guidelines, commercial or otherwise.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alexander, T., Greco, R. Hematopoietic stem cell transplantation and cellular therapies for autoimmune diseases: overview and future considerations from the Autoimmune Diseases Working Party (ADWP) of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant 57, 1055–1062 (2022). https://doi.org/10.1038/s41409-022-01702-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01702-w
This article is cited by
-
Regulatory T cells expressing CD19-targeted chimeric antigen receptor restore homeostasis in Systemic Lupus Erythematosus
Nature Communications (2024)
-
Loss of NLRP6 expression increases the severity of intestinal injury after syngeneic hematopoietic stem cell transplantation
Annals of Hematology (2024)
-
Update on VEXAS and role of allogeneic bone marrow transplant: Considerations on behalf of the Chronic Malignancies Working Party of the EBMT
Bone Marrow Transplantation (2022)